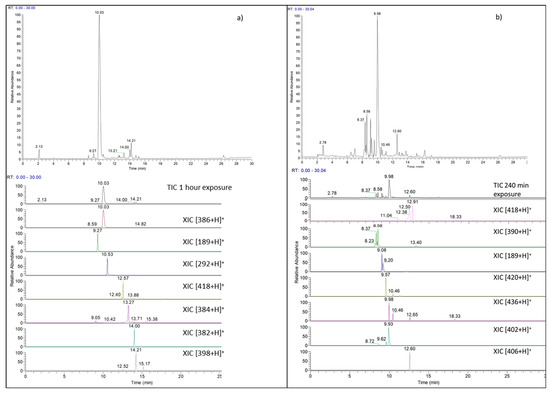
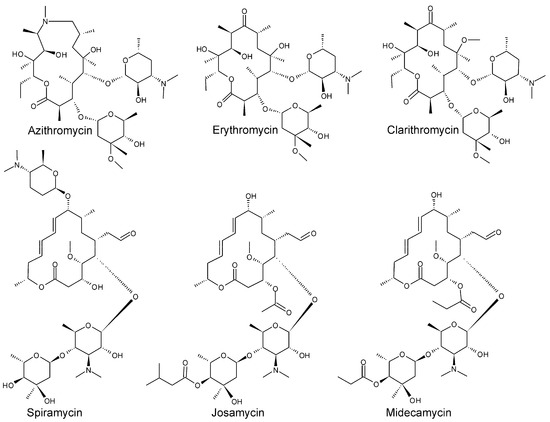
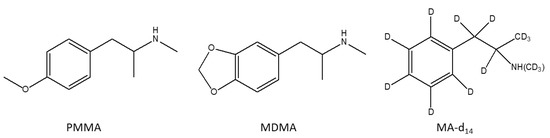
Recent Trend in Chromatography for Pharmaceutical Analysis (Closed)
A topical collection in Chemosensors (ISSN 2227-9040). This collection belongs to the section "Analytical Methods, Instrumentation and Miniaturization".
Viewed by 43976Editors
2. UCIBIO—Applied Molecular Biosciences Unit, Translational Toxicology Research Laboratory, University Institute of Health Sciences (1H-TOXRUN, IUCS-CESPU), 4585-116 Gandra, Portugal
Interests: chromatography; chiral drugs; pharmaceuticals; environmental contaminants
Special Issues, Collections and Topics in MDPI journals
2. TOXRUN–Toxicology Research Unit, University Institute of Health Sciences, CESPU, CRL, 4585-116 Gandra, Portugal
Interests: organic and pharmaceutical chemistry; chromatography; chirality; organic environmental pollutants
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear colleagues,
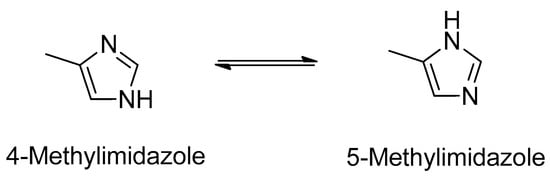
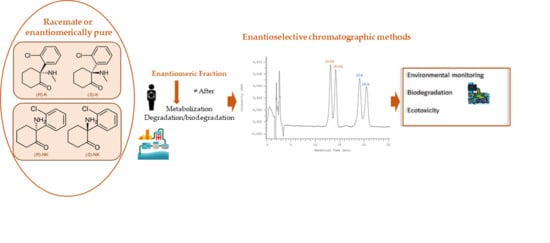
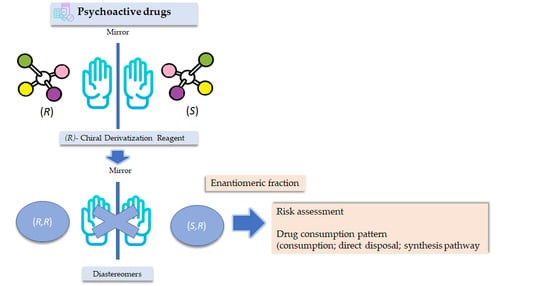
Pharmaceuticals are synthetic or natural chemicals that can be found in prescription medicines, over-the-counter therapeutic drugs, and veterinary drugs. Nevertheless, due to their biological properties, use of off-label pharmaceuticals and their misuse is widespread (e.g., in aquaculture, agriculture, doping in sport). Therefore, the separation, detection, and quantification of pharmaceuticals is required in different areas. Further, many pharmaceuticals are chiral and, thus, enantiomeric analysis is required in many fields and for different purposes.
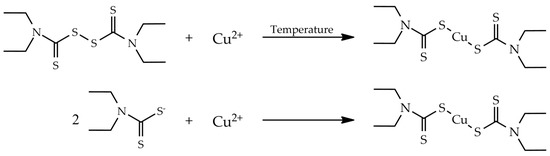
Chromatography is the elected methodology for routine and research laboratories for the analysis of pharmaceuticals and their metabolites using both non-enantioselective and enantioselective methods. It is among the most advanced and versatile methodology concerning equipment, stationary phases, and chromatographic elution modes allowing good selectivity/specificity, short analysis time, and beyond accurate and precise data.
A variety of chromatographic techniques coupled with different detectors have been developed for both preparation and analysis of pharmaceuticals. For example, in manufacturing processes to assess the stability of drugs and test for impurities and degradation products as well as in clinical analysis for pharmacokinetic studies or therapeutic monitoring. However, chromatographic methods for the analysis of pharmaceuticals have also been reported in other fields such as forensic toxicology and food and environmental analysis.
Therefore, this Topical Collection aims to focus on recent and innovative preparative and analytical chromatographic methods used for the analysis of pharmaceuticals in different areas covering industry, clinical and medical purposes, forensic toxicology, and food and environmental analysis.
Prof. Dr. Cláudia Maria Rosa Ribeiro
Prof. Dr. Maria Elizabeth Tiritan
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Chemosensors is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- chromatography
- pharmaceuticals
- industry
- forensic toxicology
- food analysis
- environment