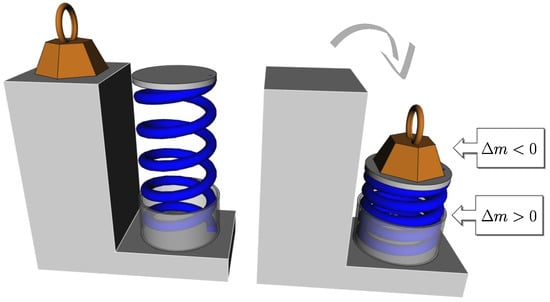
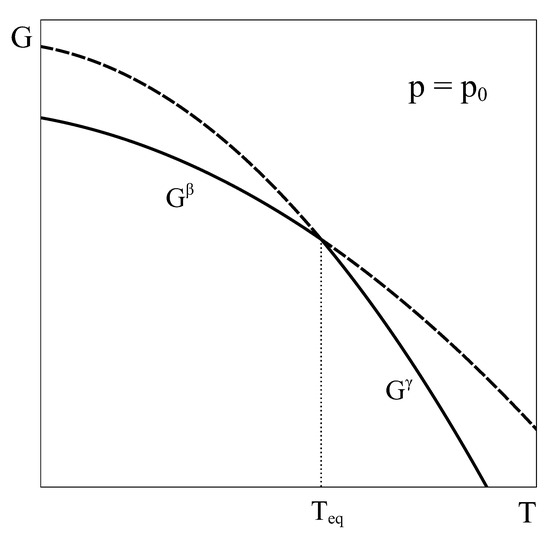
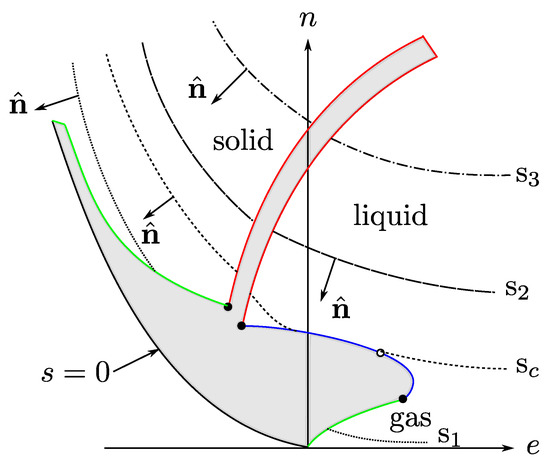
Foundations and Ubiquity of Classical Thermodynamics
A topical collection in Entropy (ISSN 1099-4300). This collection belongs to the section "Thermodynamics".
Viewed by 40510Editor
Interests: fundamental laws of nature; thermodynamics and heat transfer fundamentals; the second law of thermodynamics and entropy; energy efficiency; conservation and sustainability; fluids-thermal-energy components and systems; nanotechnology and nanofluids
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Thermodynamics is a science of Energy and Entropy, and therefore, the very foundation of all other sciences and of all creative applications.
Classical thermodynamics crystallizes a diverse and complex reality to a fundamental cause-and-effect ubiquitous simplicity by its fundamental principles. That is why it is hard to understand thermodynamics “the first time or the second time through”.
The phenomenological Laws of Thermodynamics have much wider, including philosophical, significance and implications than their simple expressions based on experimental observations—they are The Fundamental Laws of Nature: The Zeroth (equilibrium existentialism), the First (conservational transformationalism), the Second (forced, irreversible–directional transformationalism), and the Third (unattainability of ‘emptiness’). These Laws define and unify our comprehension of all existence and transformations in the universe.
Classical thermodynamics, formalized in the nineteenth century by Sadi Carnot, Clausius, Joule, Helmholtz, Kelvin, Gibbs, and others, established the fundamental laws by corelating the energy of thermomechanical processes and macroproperties irrespective of the microstructure of material systems. The fundamental laws and macroscopic constitutive correlations have been deduced from reversible process-equivalency, from one to another equilibrium state, but are nevertheless conceptually universal and valid for all diverse irreversible processes at all space and time scales.
Phenomenological, classical thermodynamics, with its fundamental principles, paved the way for the development of modern thermodynamics in the twentieth century by Lars Onsager, Ilya Prigogine, and others, with branches in many areas of theoretical and practical sciences, by expanding classical, fundamental laws to the underlying microstructure of all existence and diverse processes in nature, the latter driven by free energy’s irreversible forcing and displacement of mass-energy towards mutual equilibrium uniformity, while dissipating nonequilibrium free energy to thermal heat and thus expanding the thermal displacement space, quantified by the entropy production and entropy property.
From classical to chemical to biological thermodynamics, from heat engines to so-called self-evolving dissipative structures and life processes, there are many challenges and opportunities to be comprehended and further developed. Human comprehension is always subjective, and more objective progress requires that all possible alternatives be recognized and considered to advance our knowledge. The causes and effects are entwined and often hard to differentiate. The irreversible processes are destroyers but also creators of order. Are creation of order and life processes within “dissipative structures” driven by dissipative processes or are the latter mere consequences of irreversible processes driven by nonequilibrium free energy? These are but some of the many challenges and opportunities to be comprehended and creatively exploited for the betterment of the society and sustainability of nature we live in.
Classical, phenomenological thermodynamics today has unjustifiably a dubious status. Some modern physicists regard classical thermodynamics as an obsolete relic. Often, mostly due to lack of comprehension, thermodynamics is considered as an engineering subject and thus not as the most fundamental science of energy and nature. Apart from the view that thermodynamics is obsolete, there is a widespread belief among scientists in thermodynamics’ absolute authority.
This Topical Collection focuses on original reasoning and new research results in fundamentals and applications in thermodynamics. Original manuscripts with a focus on phenomenological fundamentals and applications, including critical up-to-date reviews, are solicited. We welcome submissions addressing novel issues, as well as those on more specific topics. It is hoped that this collection will inspire and motivate scientists and practitioners to revisit important and critical issues related to the Laws of Thermodynamics as the most fundamental laws of nature.
Prof. Dr. Milivoje M. Kostic
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Entropy is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.