Advances in Omega-3 Research
(Closed)
Share This Topical Collection
Editor
 Prof. Dr. Jing X. Kang
Prof. Dr. Jing X. Kang
 Prof. Dr. Jing X. Kang
Prof. Dr. Jing X. Kang
E-Mail
Website
Collection Editor
Laboratory for Lipid Medicine and Technology, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Interests: omega-3; omega-6/omega-3 ratio; nutrigenomics; nutri-biotechnology; cancer metabolism; lipidomics; gut microbiota; nutritional interventions; inflammation; metabolic syndrome; chronic diseases
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Omega‐3 polyunsaturated fatty acids as essential nutrients, dietary supplements, and pharmaceutical drugs have been widely investigated and used over the past few decades. The continued interest and ever‐growing volume of research in the field point to omega‐3 fatty acids as a key player in the management of chronic diseases. However, the main challenge of omega‐3 research lies in its complexity and confounding factors. Variations, inconsistencies, and controversies on the study outcomes of omega‐3 fatty acids remain to be clarified. Recent advances in analytical technologies (such as “omics”), new animal models, and integrated experimental approaches have allowed us to more comprehensively and accurately elucidate the biological effects of omega‐3 fatty acids and their mechanisms and readily identify biomarkers for efficacy evaluation. Novel concepts and emerging technologies are being used for production, process, and analysis of omega-3 fatty acids, as well as for the development of omega‐3‐based interventions for health promotion and disease management.
The field of omega-3 research has evolved rapidly in recent years, and its potential applications have become more diverse and significant for public health. The aim of this topic collection is to provide a platform for sharing research progress and highlight the frontier of the field of omega-3 fatty acids.
Prof. Jing X. Kang
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
There is an Article Processing Charge (APC) for publication in this
open access journal. For details about the APC please see here.
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- Biochemistry
- Biotechnology
- Lipidomics
- Metabolomics
- Genomics
- Proteomics
- animal models
- imaging
- gut microbiota
- inflammation
- lipogenesis
- biomarkers
- nutrition and metabolism
- translation
- dietary supplements
- therapeutics
- interventions
- disease prevention and treatment
- brain development
- maternal and vhild health
- metabolic syndrome
- chronic diseases
- aging
Published Papers (5 papers)
Open AccessArticle
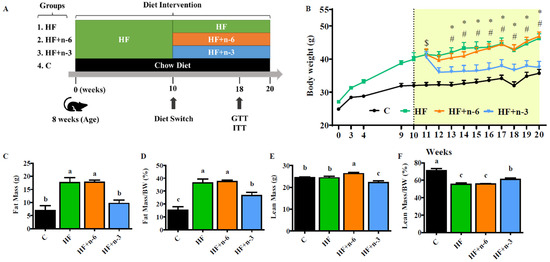
Differential Interventional Effects of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on High Fat Diet-Induced Obesity and Hepatic Pathology
by
Lei Hao, Chih-Yu Chen, Yong-Hui Nie, Kanakaraju Kaliannan and Jing X. Kang
Cited by 2 | Viewed by 2390
Abstract
Current Dietary Guidelines for Americans recommend replacing saturated fat (SFA) intake with polyunsaturated fatty acids (PUFAs) and monosaturated fatty acids (MUFAs) but do not specify the type of PUFAs, which consist of two functionally distinct classes: omega-6 (n-6) and omega-3 (n-3) PUFAs. Given
[...] Read more.
Current Dietary Guidelines for Americans recommend replacing saturated fat (SFA) intake with polyunsaturated fatty acids (PUFAs) and monosaturated fatty acids (MUFAs) but do not specify the type of PUFAs, which consist of two functionally distinct classes: omega-6 (n-6) and omega-3 (n-3) PUFAs. Given that modern Western diets are already rich in n-6 PUFAs and the risk of chronic disease remains high today, we hypothesized that increased intake of n-3 PUFAs, rather than n-6 PUFAs, would be a beneficial intervention against obesity and related liver diseases caused by high-fat diets. To test this hypothesis, we fed C57BL/6J mice with a high-fat diet (HF) for 10 weeks to induce obesity, then divided the obese mice into three groups and continued feeding for another 10 weeks with one of the following three diets: HF, HF+n-6 (substituted half of SFA with n-6 PUFAs), and HF+n-3 (substituted half of SFA with n-3 PUFAs), followed by assessment of body weight, fat mass, insulin sensitivity, hepatic pathology, and lipogenesis. Interestingly, we found that the HF+n-6 group, like the HF group, had a continuous increase in body weight and fat mass, while the HF+n-3 group had a significant decrease in body weight and fat mass, although all groups had the same calorie intake. Accordingly, insulin resistance and fatty liver pathology (steatosis and fat levels) were evident in the HF+n-6 and HF groups but barely seen in the HF+n-3 group. Furthermore, the expression of lipogenesis-related genes in the liver was upregulated in the HF+n-6 group but downregulated in the HF+n-3 group. Our findings demonstrate that n-6 PUFAs and n-3 PUFAs have differential effects on obesity and fatty liver disease and highlight the importance of increasing n-3 PUFAs and reducing n-6 PUFAs (balancing the n-6/n-3 ratio) in clinical interventions and dietary guidelines for the management of obesity and related diseases.
Full article
►▼
Show Figures
Open AccessArticle
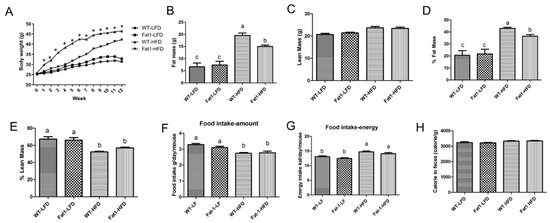
Omega-3 Polyunsaturated Fatty Acids Protect against High-Fat Diet-Induced Morphological and Functional Impairments of Brown Fat in Transgenic Fat-1 Mice
by
Lei Hao, Yong-Hui Nie, Chih-Yu Chen, Xiang-Yong Li, Kanakaraju Kaliannan and Jing X. Kang
Cited by 4 | Viewed by 2406
Abstract
The role of omega-3 polyunsaturated fatty acids (n-3 PUFAs) in the regulation of energy homeostasis remains poorly understood. In this study, we used a transgenic
fat-1 mouse model, which can produce n-3 PUFAs endogenously, to investigate how n-3 PUFAs regulate the morphology and
[...] Read more.
The role of omega-3 polyunsaturated fatty acids (n-3 PUFAs) in the regulation of energy homeostasis remains poorly understood. In this study, we used a transgenic
fat-1 mouse model, which can produce n-3 PUFAs endogenously, to investigate how n-3 PUFAs regulate the morphology and function of brown adipose tissue (BAT). We found that high-fat diet (HFD) induced a remarkable morphological change in BAT, characterized by “whitening” due to large lipid droplet accumulation within BAT cells, associated with obesity in wild-type (WT) mice, whereas the changes in body fat mass and BAT morphology were significantly alleviated in
fat-1 mice. The expression of thermogenic markers and lypolytic enzymes was significantly higher in
fat-1 mice than that in WT mice fed with HFD. In addition,
fat-1 mice had significantly lower levels of inflammatory markers in BAT and lipopolysaccharide (LPS) in plasma compared with WT mice. Furthermore,
fat-1 mice were resistant to LPS-induced suppression of UCP1 and PGC-1 expression and lipid deposits in BAT. Our data has demonstrated that high-fat diet-induced obesity is associated with impairments of BAT morphology (whitening) and function, which can be ameliorated by elevated tissue status of n-3 PUFAs, possibly through suppressing the effects of LPS on inflammation and thermogenesis.
Full article
►▼
Show Figures
Open AccessCommentary
Potential Suicide Prophylactic Activity by the Fish Oil Metabolite, 4-Hydroxyhexenal
by
Hans O. Kalkman
Cited by 1 | Viewed by 1603
Abstract
Low levels of n-3 poly-unsaturated fatty acids (n-3 PUFAs) and high levels of n-6 PUFAs in the blood circulation are associated with an increased risk for suicide. Clinical studies indicate that docosahexaenoic acid (DHA, a n-3 PUFA found in fish-oil) displays protective effects
[...] Read more.
Low levels of n-3 poly-unsaturated fatty acids (n-3 PUFAs) and high levels of n-6 PUFAs in the blood circulation are associated with an increased risk for suicide. Clinical studies indicate that docosahexaenoic acid (DHA, a n-3 PUFA found in fish-oil) displays protective effects against suicide. It has recently been proposed that the activation of the transcription factor NRF2 might be the pharmacological activity that is common to current anti-suicidal medications. Oxidation products from fish oil, including those from DHA, are electrophiles that reversibly bind to a protein ‘KEAP1’, which acts as the molecular inhibitor of NRF2 and so indirectly promotes NRF2-transcriptional activity. In the majority of publications, the NRF2-stimulant effect of DHA is ascribed to the metabolite 4-hydroxyhexenal (4HHE). It is suggested to investigate whether 4HHE will display a therapeutically useful anti-suicidal efficacy.
Full article
Open AccessArticle
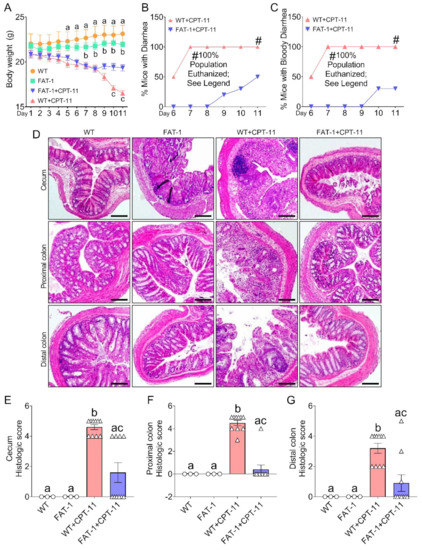
Decreased Tissue Omega-6/Omega-3 Fatty Acid Ratio Prevents Chemotherapy-Induced Gastrointestinal Toxicity Associated with Alterations of Gut Microbiome
by
Kanakaraju Kaliannan, Shane O. Donnell, Kiera Murphy, Catherine Stanton, Chao Kang, Bin Wang, Xiang-Yong Li, Atul K. Bhan and Jing X. Kang
Cited by 7 | Viewed by 3461
Abstract
Gastrointestinal toxicity (GIT) is a debilitating side effect of Irinotecan (CPT-11) and limits its clinical utility. Gut dysbiosis has been shown to mediate this side effect of CPT-11 by increasing gut bacterial β-glucuronidase (GUSB) activity and impairing the intestinal mucosal barrier (IMB). We
[...] Read more.
Gastrointestinal toxicity (GIT) is a debilitating side effect of Irinotecan (CPT-11) and limits its clinical utility. Gut dysbiosis has been shown to mediate this side effect of CPT-11 by increasing gut bacterial β-glucuronidase (GUSB) activity and impairing the intestinal mucosal barrier (IMB). We have recently shown the opposing effects of omega-6 (n-6) and omega-3 (n-3) polyunsaturated fatty acids (PUFA) on the gut microbiome. We hypothesized that elevated levels of tissue n-3 PUFA with a decreased n-6/n-3 PUFA ratio would reduce CPT-11-induced GIT and associated changes in the gut microbiome. Using a unique transgenic mouse (FAT-1) model combined with dietary supplementation experiments, we demonstrate that an elevated tissue n-3 PUFA status with a decreased n-6/n-3 PUFA ratio significantly reduces CPT-11-induced weight loss, bloody diarrhea, gut pathological changes, and mortality. Gut microbiome analysis by 16S rRNA gene sequencing and QIIME2 revealed that improvements in GIT were associated with the reduction in the CPT-11-induced increase in both GUSB-producing bacteria (e.g.,
Enterobacteriaceae) and GUSB enzyme activity, decrease in IMB-maintaining bacteria (e.g.,
Bifidobacterium), IMB dysfunction and systemic endotoxemia. These results uncover a host–microbiome interaction approach to the management of drug-induced gut toxicity. The prevention of CPT-11-induced gut microbiome changes by decreasing the tissue n-6/n-3 PUFA ratio could be a novel strategy to prevent chemotherapy-induced GIT.
Full article
►▼
Show Figures
Open AccessArticle
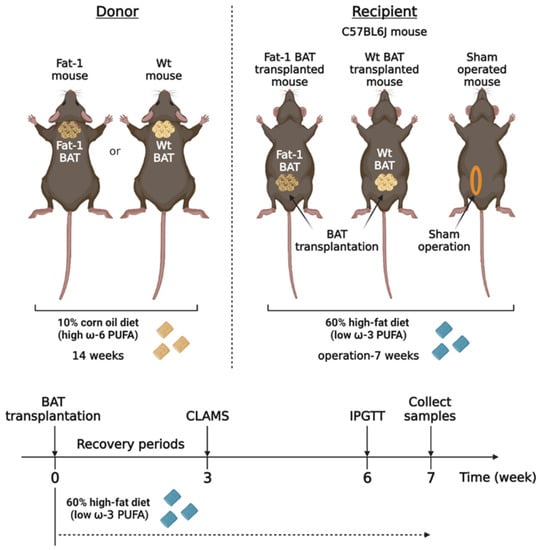
Transplantation of Brown Adipose Tissue with the Ability of Converting Omega-6 to Omega-3 Polyunsaturated Fatty Acids Counteracts High-Fat-Induced Metabolic Abnormalities in Mice
by
Tadataka Tsuji, Valerie Bussberg, Allison M. MacDonald, Niven R. Narain, Michael A. Kiebish and Yu-Hua Tseng
Cited by 5 | Viewed by 4962
Abstract
A balanced omega (ω)-6/ω-3 polyunsaturated fatty acids (PUFAs) ratio has been linked to metabolic health and the prevention of chronic diseases. Brown adipose tissue (BAT) specializes in energy expenditure and secretes signaling molecules that regulate metabolism via inter-organ crosstalk. Recent studies have uncovered
[...] Read more.
A balanced omega (ω)-6/ω-3 polyunsaturated fatty acids (PUFAs) ratio has been linked to metabolic health and the prevention of chronic diseases. Brown adipose tissue (BAT) specializes in energy expenditure and secretes signaling molecules that regulate metabolism via inter-organ crosstalk. Recent studies have uncovered that BAT produces different PUFA species and circulating oxylipin levels are correlated with BAT-mediated energy expenditure in mice and humans. However, the impact of BAT ω-6/ω-3 PUFAs on metabolic phenotype has not been fully elucidated. The Fat-1 transgenic mice can convert ω-6 to ω-3 PUFAs. Here, we demonstrated that mice receiving Fat-1 BAT transplants displayed better glucose tolerance and higher energy expenditure. Expression of genes involved in thermogenesis and nutrient utilization was increased in the endogenous BAT of mice receiving Fat-1 BAT, suggesting that the transplants may activate recipients’ BAT. Using targeted lipidomic analysis, we found that the levels of several ω-6 oxylipins were significantly reduced in the circulation of mice receiving Fat-1 BAT transplants than in mice with wild-type BAT transplants. The major altered oxylipins between the WT and Fat-1 BAT transplantation were ω-6 arachidonic acid-derived oxylipins via the lipoxygenase pathway. Taken together, these findings suggest an important role of BAT-derived oxylipins in combating obesity-related metabolic disorders.
Full article
►▼
Show Figures