Biomarker for Non-small Cell Lung Cancer: From the Bench to the Bedside
A topical collection in Journal of Clinical Medicine (ISSN 2077-0383). This collection belongs to the section "Oncology".
Viewed by 46758Editor
2. Division of Medical Oncology, Escola Paulista de Medicina, Federal University of São Paulo, São Paulo, Brazil
3. Algarve Biomedical Centre, Department of Biomedical Sciences and Medicine, University of Algarve, Faro, Portugal
Interests: immunotherapy; lung cancer; targeted therapy; predictive biomarkers; prognostic biomarker
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
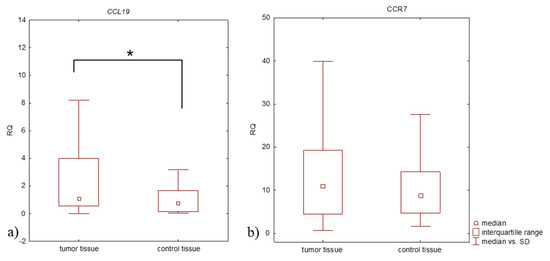
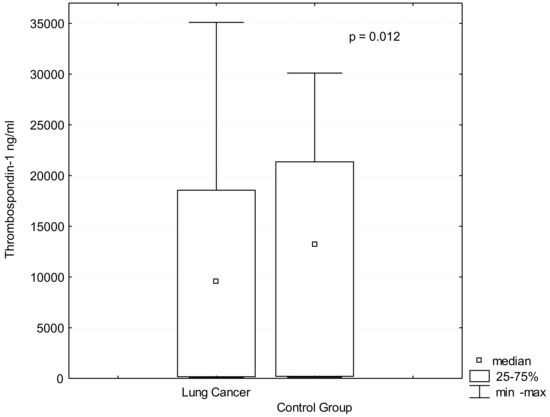
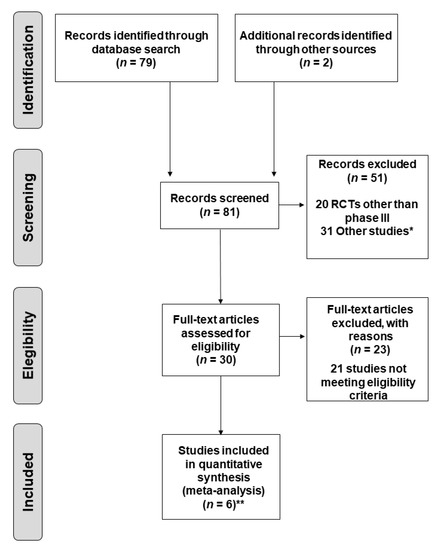
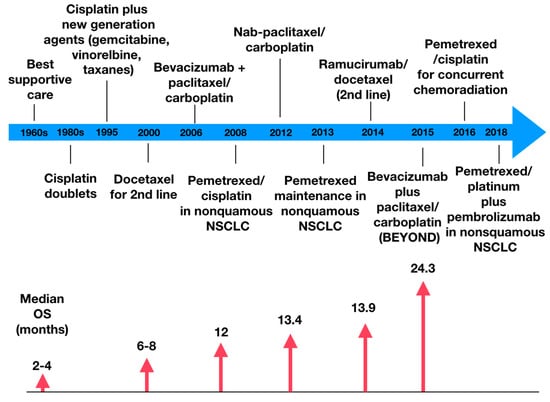
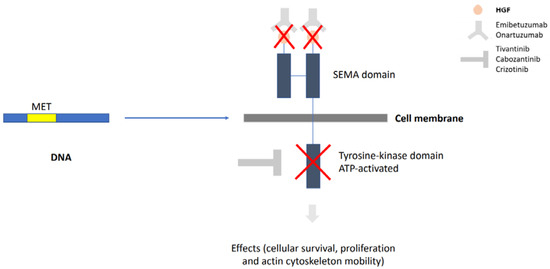
Lung cancer is a very aggressive disease. Currently, personalizing medicine has acquired a main role in the clinical practice in order to tailor the best treatment for advanced non-small lung cancer patients (NSCLC). Deeply understanding the molecular mechanisms of NSCLC is very important to the development of new drugs and biomarkers through inhibiting driver mutations responsible for disease pathogenesis. Thus, we believe that this Special Issue will be important to publish the latest updated research in NSCLC regarding future trends in prognostic and predictive biomarkers to help oncologists in the provision of the best care. All types of articles will be considered, such as narrative reviews, meta-analyses, original articles, commentaries and editorials.
Prof. Dr. Ramon Andrade De Mello
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Journal of Clinical Medicine is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- TIM3
- Lung cancer
- Targeted therapies
- Immunetherapy
- Predictive biomarkers
- Prognostic biomarkers