Synthesis and Antifungal Activity of Novel Triazole Compounds Containing Piperazine Moiety
Abstract
:1. Introduction


2. Results and Discussion
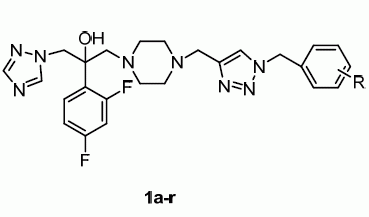
2.1. Chemistry
2.2. Pharmacology

| Compd | C. alb14053 | C. alb. 20352 | C. par. | C. neo. | C. gla. | A. fum. | T. rub. | M. gyp. |
|---|---|---|---|---|---|---|---|---|
| 1a | 2 | 8 | 4 | 8 | >64 | >64 | 8 | 4 |
| 1b | 1 | 4 | 4 | 4 | 4 | >64 | 4 | 16 |
| 1c | 1 | 2 | 8 | 2 | 4 | >64 | 8 | 8 |
| 1d | 0.25 | 0.5 | 1 | 1 | 16 | >64 | 2 | 8 |
| 1e | 1 | 1 | 1 | 1 | 16 | >64 | 1 | 4 |
| 1f | 0.5 | 0.5 | 1 | 1 | >64 | >64 | 1 | 1 |
| 1g | 0.5 | 0.5 | 1 | 1 | 16 | >64 | 1 | 0.5 |
| 1h | 0.5 | 0.5 | 1 | 0.5 | 32 | >64 | 1 | 0.5 |
| 1i | 0.25 | 0.5 | 0.5 | 0.5 | >64 | >64 | 1 | 0.5 |
| 1j | 1 | 2 | 4 | 4 | >64 | >64 | 4 | 0.25 |
| 1k | 64 | 64 | 4 | 4 | >64 | >64 | 4 | 0.25 |
| 1l | 1 | 4 | 4 | 0.5 | 64 | >64 | 1 | 0.25 |
| 1m | 4 | 16 | 16 | 16 | >64 | >64 | 16 | 16 |
| 1n | 8 | 8 | 16 | 8 | >64 | >64 | 16 | 4 |
| 1o | 8 | 8 | 16 | 8 | >64 | >64 | 16 | 2 |
| 1p | 2 | 4 | 8 | 4 | >64 | >64 | 4 | 2 |
| 1q | 1 | 8 | 8 | 8 | >64 | >64 | 4 | 2 |
| 1r | 2 | 8 | 8 | 4 | 64 | >64 | 4 | 0.25 |
| ICZ | 0.0625 | 0.0625 | 0.03125 | 0.0156 | 0.5 | 2 | 0.125 | 4 |
| VCZ | 0.03125 | 0.0625 | 0.03125 | 0.0156 | 0.5 | 0.25 | 0.0625 | 0.25 |
| FCZ | 1 | 0.5 | 0.5 | 0.5 | 2 | >64 | 4 | 64 |
3. Experimental Section
3.1. General Procedures
3.2. General Procedure for the Preparation of the Compounds 1a–r
4. Conclusions
Acknowledgments
Supplementary Materials
Supplementary Files
Supplementary File 1Author Contributions
Conflicts of Interest
References and Notes
- Gadhave, P.P.; Dighe, N.S.; Pattan, S.R.; Deotarse, P.; Musmade, D.S.; Shete, R. Current biological and synthetic profile of triazoles: A review. Annals Biol. Res. 2010, 1, 82–89. [Google Scholar]
- Groll, A.H.; Lumb, J. New developments in invasive fungal disease. Future Microbiol. 2012, 7, 179–184. [Google Scholar] [CrossRef]
- Casalinuovo, I.A.; di Francesco, P.; Garaci, E. Fluconazole resistance in Candida albicans: A review of mechanisms. Eur. Rev. Med. Pharmacol. 2004, 8, 69–77. [Google Scholar]
- Hoffman, H.L.; Ernst, E.J.; Klepser, M.E. Novel triazole antifungal agents. Expert Opin. Inv. Drug 2000, 9, 593–605. [Google Scholar] [CrossRef]
- Aoyama, Y.; Yoshida, Y.; Sato, R. Yeast cytochrome P-450 catalyzing lanosterol 14 alpha-demethylation. II. Lanosterol metabolism by purified P-450(14)DM and by intact microsomes. J. Biol. Chem. 1984, 259, 1661–1666. [Google Scholar]
- Sheng, C.Q.; Zhang, W.N.; Ji, H.T.; Song, Y.L.; Zhang, M.; Zhou, Y.J.; Lu, J.G.; Zhu, J. Design, synthesis and antifungal activity of novel triazole derivatives. Chin. Chem. Lett. 2004, 15, 404–407. [Google Scholar]
- Kelly, S.; Arnoldi, A.; Kelly, D. Molecular genetic analysis of azole antifungal mode of action. Biochem. Soc. Trans. 1993, 21, 1034–1038. [Google Scholar]
- Sheng, C.Q.; Zhang, W.N.; Ji, H.T.; Zhang, M.; Song, Y.L.; Xu, H.; Zhu, J.; Miao, Z.Y.; Jiang, Q.F.; Yao, J.Z.; et al. Structure-based optimization of azole antifungal agents by CoMFA, CoMSIA, and molecular docking. J. Med. Chem. 2006, 49, 2512–2525. [Google Scholar] [CrossRef]
- Boyle, F.T.; Gilman, D.J.; Gravestock, M.B.; Wardleworth, J.M. Synthesis and structure-activity relationships of a novel antifungal agent, ICI 195,739. Ann. NY Acad. Sci. 1988, 544, 86–100. [Google Scholar] [CrossRef]
- Zou, Y.; Yu, S.; Li, R.; Zhao, Q.; Li, X.; Wu, M.; Huang, T.; Chai, X.; Hu, H.; Wu, Q. Synthesis, antifungal activities and molecular docking studies of novel 2-(2,4-difluorophenyl)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl) propyl dithiocarbamates. Eur. J. Med. Chem. 2014, 74, 366–374. [Google Scholar] [CrossRef]
- Yu, S.; Wang, L.; Wang, Y.; Song, Y.; Cao, Y.; Jiang, Y.; Sun, Q.; Wu, Q. Molecular docking, design, synthesis and antifungal activity study of novel triazole derivatives containing the 1,2,3-triazole group. RSC Advan. 2013, 3, 13486–13490. [Google Scholar] [CrossRef]
- Yu, S.; Chai, X.; Wang, N.; Cui, H.; Zhao, Q.; Hu, H.; Zou, Y.; Sun, Q.; Wu, Q. Synthesis and antifungal activity of the novel triazole compounds. Med. Chem. Commun. 2013, 4, 704–708. [Google Scholar] [CrossRef]
- Wang, N.; Chai, X.; Chen, Y.; Zhang, L.; Li, W.; Gao, Y.; Bi, Y.; Yu, S.; Meng, Q. Synthesis, antifungal activity, and molecular docking studies of novel triazole derivatives. Med. Chem. 2013, 9, 384–388. [Google Scholar] [CrossRef]
- Chai, X.; Yu, S.; Jiang, Y.; Zou, Y.; Wu, Q.; Zhang, D.; Jiang, Y.; Cao, Y.; Sun, Q. Design, synthesis, and biological evaluation of novel 1,2,4-triazole derivatives as antifungal agent. Arch. Pharm. Res. 2012, 35, 1895–1901. [Google Scholar] [CrossRef]
- Wang, B.G.; Yu, S.C.; Chai, X.Y.; Yan, Y.Z.; Hu, H.G.; Wu, Q.Y. Design synthesis and biological evaluation of 3-substituted triazole derivatives. Chin. Chem. Lett. 2011, 22, 519–522. [Google Scholar] [CrossRef]
- Yu, S.; Chai, X.; Hu, H.; Yan, Y.; Guan, Z.; Zou, Y.; Sun, Q.; Wu, Q. Synthesis and antifungal evaluation of novel triazole derivatives as inhibitors of cytochrome P450 14 alpha-demethylase. Eur. J. Med. Chem. 2010, 45, 4435–4445. [Google Scholar] [CrossRef]
- Zhang, X.J.; Li, H.Y.; You, L.F.; Tang, Y.; Hsung, R.P. Copper salt-catalyzed azide-[3 + 2] cycloadditions of ynamides and bis-ynamides. Adv. Synth. Catal. 2006, 348, 2437–2442. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts Approved standard; Document M27-A2; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.; Xu, K.; Bai, G.; Huang, L.; Wu, Q.; Pan, W.; Yu, S. Synthesis and Antifungal Activity of Novel Triazole Compounds Containing Piperazine Moiety. Molecules 2014, 19, 11333-11340. https://doi.org/10.3390/molecules190811333
Wang Y, Xu K, Bai G, Huang L, Wu Q, Pan W, Yu S. Synthesis and Antifungal Activity of Novel Triazole Compounds Containing Piperazine Moiety. Molecules. 2014; 19(8):11333-11340. https://doi.org/10.3390/molecules190811333
Chicago/Turabian StyleWang, Yanwei, Kehan Xu, Guojing Bai, Lei Huang, Qiuye Wu, Weihua Pan, and Shichong Yu. 2014. "Synthesis and Antifungal Activity of Novel Triazole Compounds Containing Piperazine Moiety" Molecules 19, no. 8: 11333-11340. https://doi.org/10.3390/molecules190811333
APA StyleWang, Y., Xu, K., Bai, G., Huang, L., Wu, Q., Pan, W., & Yu, S. (2014). Synthesis and Antifungal Activity of Novel Triazole Compounds Containing Piperazine Moiety. Molecules, 19(8), 11333-11340. https://doi.org/10.3390/molecules190811333