Synthesis and Biological Evaluation of Novel 6-Hydroxy-benzo[d][1,3]oxathiol-2-one Schiff Bases as Potential Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

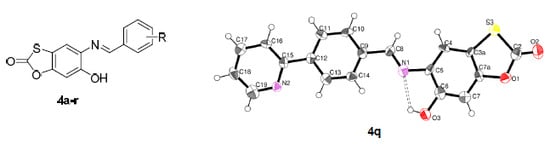
2.2. Crystallography
| Compound | MTT | Hemolysis | ||
|---|---|---|---|---|
| IC50 μM | ||||
| ACP-03 | SKMEL-19 | HCT-116 | EC50 (μg/mL) b | |
| 2 | >10 | >10 | >10 | >200 |
| 3 | >10 | >10 | >10 | >200 |
| 4a | >10 | >10 | >10 | >200 |
| 4b | 4.8 (3.2–7.2) | >10 | >10 | >200 |
| 4c | >10 | >10 | >10 | >200 |
| 4d | >10 | >10 | >10 | >200 |
| 4e | >10 | >10 | >10 | >200 |
| 4f | >10 | >10 | >10 | >200 |
| 4g | >10 | >10 | >10 | >200 |
| 4h | >10 | >10 | >10 | >200 |
| 4i | >10 | >10 | >10 | >200 |
| 4j | >10 | >10 | >10 | >200 |
| 4k | >10 | >10 | >10 | >200 |
| 4l | >10 | >10 | >10 | >200 |
| 4m | >10 | 9.4 (7.3–12.1) | >10 | >200 |
| 4n | >10 | 5.6 (4.7–6.4) | >10 | >200 |
| 4o | >10 | 2.8 (2.0–3.8) | >10 | >200 |
| 4p | >10 | >10 | >10 | >200 |
| 4q | >10 | >10 | >10 | >200 |
| 4r | >10 | >10 | >10 | >200 |
| Dox | 0.274 (0.22–0.33) | 0.045 (0.01–0.15) | 0.1 (0.05–0.28) | >200 |

2.3. Molecular Modeling

2.4. Biological Activity
| MW (Da) | PSA (A2) | HBA | HBD | ClogP | |
|---|---|---|---|---|---|
| 2 | 213.169 | 74.763 | 6 | 1 | 2.05 |
| 3 | 183.187 | 64.793 | 4 | 2 | 1.21 |
| 4a | 296.31 | 60.46 | 5 | 1 | 3.91 |
| 4b | 316.29 | 83.96 | 7 | 1 | 3.91 |
| 4c | 316.29 | 83.92 | 7 | 1 | 3.91 |
| 4d | 350.19 | 47.81 | 4 | 1 | 4.71 |
| 4e | 350.19 | 45.05 | 4 | 1 | 4.71 |
| 4f | 305.74 | 45.09 | 4 | 1 | 4.44 |
| 4g | 340.19 | 43.30 | 4 | 1 | 5.00 |
| 4h | 271.30 | 45.12 | 4 | 1 | 3.88 |
| 4i | 314.37 | 45.92 | 5 | 1 | 4.16 |
| 4j | 301.32 | 52.09 | 5 | 1 | 3.75 |
| 4k | 287.30 | 60.01 | 5 | 2 | 3.49 |
| 4l | 303.29 | 77.42 | 6 | 3 | 3.10 |
| 4m | 303.29 | 78.77 | 6 | 3 | 3.10 |
| 4n | 303.29 | 79.36 | 6 | 3 | 3.10 |
| 4o | 332.29 | 98.31 | 8 | 2 | 3.52 |
| 4p | 366.19 | 61.31 | 5 | 2 | 4.32 |
| 4q | 348.38 | 51.36 | 5 | 1 | 4.64 |
| 4r | 315.31 | 61.28 | 6 | 1 | 3.66 |
| Dox | 543.53 | 156.88 | 12 | 5 | −0.68 |
3. Experimental Section
3.1. General Information
3.2. Synthesis of 6-Hydroxy-5-nitrobenzo[d][1,3]oxathiol-2-one (2)
3.3. Synthesis of 5-Amino-6-hydroxybenzo[d][1,3]oxathiol-2-one (3)
3.4. General Procedure for Synthesis of Schiff Bases 4a–r
3.5. Cytotoxicity against Cancer Cell Lines
3.6. Cell Membrane Disruption
3.7. Molecular Modeling
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Instituto Nacional de Câncer. Instituto Nacional do Câncer. Available online: http://www1.inca.gov.br/vigilancia/ (accessed on 6 August 2014).
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/index.html (accessed on 6 August 2014).
- Caleta, I.; Kralj, M.; Marjanovic, M.; Bertosa, B.; Tomic, S.; Pavilovic, G.; Pavelic, K.; Karminski-Zamola, G. Novel cyano- and amidinobenzothiazole derivatives: Synthesis, antitumor evaluation, and X-ray and quantitative structure—Activity relationship (QSAR) analysis. J. Med. Chem. 2009, 52, 1744–1756. [Google Scholar] [CrossRef] [PubMed]
- Vellasco Júnior, W.T.; Gomes, C.R.B.; Vasconcelos, T.R.A. Chemistry and biological activities of 1,3-benzoxathiol-2-ones. Mini-Rev. Org. Chem. 2011, 8, 103–109. [Google Scholar]
- Shadyro, O.I.; Timoshchuk, V.A.; Polozov, G.I.; Povalishev, V.N.; Andreeva, O.T.; Zhelobkovich, V.E. Synthesis and antiviral activity of spatially-screened phenols: 1,3-Benzoxathiolan-2-one derivatives. Pharm. Chem. J. 1999, 33, 366–369. [Google Scholar] [CrossRef]
- Konieczny, M.T.; Konieczny, W.; Sabisz, M.; Skladanowski, A.; Wakieć, R.; Augustynowicz-Kopeć, E.; Zwolska, S. Synthesis of isomeric, oxathiolone fused chalcones, and comparison of their activity toward various microorganisms and human cancer cells line. Chem. Pharm. Bull. 2007, 55, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, M.T.; Konieczny, W.; Sabisz, M.; Skladanowski, A.; Wakieć, R.; Augustynowicz-Kopeć, E.; Zwolska, S. Acid-catalyzed synthesis of oxathiolone fused chalcones. Comparison of their activity toward various microorganisms and human cancer cells line. Eur. J. Med. Chem. 2007, 42, 729–733. [Google Scholar]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.; de Resende, M.A.; Martins, C.V.B.; de Fátima, A. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar]
- Rana, K.; Pandurangan, A.; Singh, N.; Tiwari, A.K. A systemic review of schiff bases as an analgesic, anti-inflammatory. Int. J. Curr. Pharm. Res. 2012, 4, 5–11. [Google Scholar]
- Ferreira, M.L.; Vasconcelos, T.R.A.; de Carvalho, E.M.; Lourenço, M.C.S.; Wardell, S.M.S.V.; Wardell, J.L.; Ferreira, V.F.; de Souza, M.V.N. Synthesis and antitubercular activity of novel Schiff bases derived from d-mannitol. Carbohydr. Res. 2009, 344, 2042–2047. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ge, H.-M.; Tan, S.-H.; Li, H.-Q.; Song, Y.-C.; Zhu, H.-L.; Tan, R.-X. Synthesis and antimicrobial activities of Schiff bases derived from 5-chloro-salicylaldehyde. Eur. J. Med. Chem. 2007, 42, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Patil, V.M.; Sharma, V.K.; Khosa, R.L.; Masand, N. Schiff bases: A review on biological insights. Int. J. Drug Des. Discov. 2012, 3, 851–868. [Google Scholar]
- Junior, I.N.; Lourenço, M.C.S.; Henriques, M.G.M.O.; Ferreira, B.; Vasconcelos, T.R.A.; Peralta, M.A.; de Oliveira, P.S.M.; Wardell, S.M.S.V.; de Souza, M.V.N. Synthesis and anti-mycobacterial activity of N'-[(E)-(disubstituted-phenyl)methylidene]isonicotino-hydrazide derivatives. Lett. Drug Des. Discov. 2005, 2, 563–566. [Google Scholar] [CrossRef]
- Wardell, S.M.S.V.; de Souza, M.V.N.; Ferreira, M.L.; Vasconcelos, T.R.A.; Low, J.N.; Glidewell, C. Pi-Stacked hydrogen-bonded chains of rings in 2,4-difluorobenzaldehyde isonicotinoylhydrazone and hydrogen-bonded sheets in 2,3-dichlorobenzaldehyde isonicotinoylhydrazone. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2005, 61, 617–620. [Google Scholar] [CrossRef]
- Lourenço, M.C.S.; Ferreira, M.L.; de Souza, M.V.N.; Peralta, M.A.; Vasconcelos, T.R.A.; Henriques, M.G.M.O. Synthesis and anti-mycobacterial activity of (E)-N'-(monosubstituted-benzylidene)isonicotinohydrazide derivatives. Eur. J. Med. Chem. 2008, 43, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.F.; Azevedo, E.C.; Ferreira, V.F.; Araújo, A.J.; Santos, E.A.; Pessoa, C.; Costa-Lotufo, L.V.; Montenegro, R.C.; Moraes, M.O.; Vasconcelos, T.R.A.; et al. Synthesis and antitumor evaluation of (E)-2-benzothiazole hydrazones. Lett. Drug Des. Discov. 2010, 7, 551–555. [Google Scholar]
- Nogueira, A.F.; Vasconcelos, T.R.A.; Wardell, J.L.; Wardell, S.M.S.V. Crystal structures of hydrazones, 2-(1,3-benzothiazolyl)-NH-N=CH-Ar, prepared from arenealdehydes and 2-hydrazinyl-1,3-benzothiazole. Z. Kristallogr. Cryst. Mater. 2011, 226, 846–860. [Google Scholar] [CrossRef]
- Lindgren, E.B.; Yoneda, J.D.; Leal, K.Z.; Nogueira, A.F.; Vasconcelos, T.R.A.; Wardell, J.L.; Wardell, S.M.S.V. Structures of hydrazones, (E)-2-(1,3-benzothiazolyl)-NH-N=CH-Ar, [Ar = 4-(pyridin-2-yl)phenyl, pyrrol-2-yl, thien-2-yl and furan-2-yl]: Difference in conformations and intermolecular hydrogen bonding. J. Mol. Struct. 2013, 1036, 19–27. [Google Scholar] [CrossRef]
- Reis, R.R.; Azevedo, E.C.; de Souza, M.C.B.V.; Ferreira, V.F.; Montenegro, R.C.; Araújo, A.J.; Pessoa, C.; Costa-Lotufo, L.V.; Moraes, M.O.; Filho, J.D.B.M.; et al. Synthesis and anticancer activities of some novel 2-(benzo[d]thiazol-2-yl)-8-substituted-2H-pyrazolo[4,3-c]quinolin-3(5H)-ones. Eur. J. Med. Chem. 2011, 46, 1448–1452. [Google Scholar]
- Facchinetti, V.; Reis, R.R.; Gomes, C.R.B.; Vasconcelos, T.R.A. Chemistry and biological activities of 1,3-benzothiazoles. Mini-Rev. Org. Chem. 2012, 9, 44–53. [Google Scholar] [CrossRef]
- Facchinetti, V.; Gomes, C.R.B.; de Souza, M.V.N.; Vasconcelos, T.R.A. Perspectives on the development of novel potentially active quinolones against tuberculosis and cancer. Mini-Rev. Med. Chem. 2012, 12, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.B.; Brito, M.A.; Vasconcelos, T.R.A.; Moraes, M.O.; Montenegro, R.C.; Yoneda, J.D.; Leal, K.Z. Synthesis and anticancer activity of (E)-2-benzothiazole hydrazones. Eur. J. Med. Chem. 2014, 86, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Sathunuru, R.; Rao, U.N.; Biehl, E. Facile, high-yield, regioselective synthesis of ortho-nitrophenols using cerium (IV) ammonium nitrate. ARKIVOC 2003, 2003, 124–133. [Google Scholar] [CrossRef]
- Fox, B.A.; Threlfall, T.L. 2,3-Diaminopyridine. Org. Synth. Coll. Vol. 1973, 5, 346–351. [Google Scholar]
- Bellamy, F.D.; Ou, K. Reduction of aromatic nitro compounds with stannous chloride in non-acidic and non-aqueous medium. Tetrahedron Lett. 1984, 25, 839–842. [Google Scholar] [CrossRef]
- Ramadas, K.; Srinavasan, N. Iron-ammonium chloride—A convenient and inexpensive reductant. Synth. Commun. 1992, 22, 3189–3195. [Google Scholar] [CrossRef]
- Data for compound 4q were obtained at 100(2) K with Mo-Kα radiation by means of the Rigaku Saturn724+ (2x2 bin mode). Data collection, data reduction and unit cell refinement were carried out under the control of the program CrystalClear-SM Expert 2.0 r7 [29]. Data were collected at the NCS Crystallographic Service, based at the University of Southampton. Correction for absorption was achieved by a semi-empirical method based upon the variation of equivalent reflections [29]. The programs ORTEP-3 for Windows [30] and MERCURY [31] were used in the preparation of the Figures. SHELXL97 [32] and PLATON [33] were used in the calculation of molecular geometry. The structures were solved by direct methods using SHELXS-97 [32] and fully refined by means of the program SHELXL-97 [32]. All hydrogen atoms were placed in calculated positions.
- CrystalClear; Rigaku Inc.: The Woodlands, TX, USA, 2010.
- Flack, H.D. On enantiomorph-polarity estimation. Acta Crystallogr. Sect. A Found. Crystallogr. 1983, 39, 876–881. [Google Scholar] [CrossRef]
- MERCURY 3.01; Cambridge Crystallographic Data Centre: Cambridge, UK, 2013.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Crystal data collected at 100(2)K, colourless crystal: 0.04 × 0.04 × 0.01 mm. Formula: C19H12N22O3S; M = 348.38; orthorhombic, Pbcn; a = 18.336(6) Å, b = 11.547(4) Å, c = 14.303(5) Å, Z = 8, V = 3028.3(18) Å3, independent reflections 2671 [R(int) = 0.36], 1197 observed reflections [I > 2 σ (I)]: Parameters refined 2671; number of restraints 0; R(F) 0.102 [I > 2 σ (I)], Largest diff. peak 0.36 Å_1. Atomic coordinates, bond lengths, angles and thermal parameters have been deposited at the Cambridge Crystallographic Data Centre, deposition number 984867.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- NCI/NIH Developmental Therapeutcs Program. Available online: http://dtp.nci.nih.gov/branches/btb/handlingprep.html (accessed on 20 August 2014).
- Costa-Lotufo, L.V.; Cunha, G.M.A.; Farias, P.A.M.; Viana, G.S.B.; Cunha, K.M.A.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Gramosa, N.V.; Rao, V.S.N.; et al. The cytotoxic and embryotoxic effects of kaurenoic acid, a diterpene isolated from Copaifera langsdorffii oleo-resin. Toxicon 2002, 40, 1231–1234. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Zhao, Y.; Huang, R.; Jiang, C.; Pei, Y. Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J. Pharm. Sci. 2008, 97, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Benival, D.M.; Devarajan, P.V. Lipomer of doxorubicin hydrochloride for enhanced oral bioavailability. Int. J. Pharm. 2012, 423, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Spartan’10; Wavefunction Inc.: Irvine, CA, USA, 2011.
- Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013.
- Sample Availability: Samples of the compounds 2, 3 and 4a–r are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chazin, E.D.L.; Sanches, P.D.S.; Lindgren, E.B.; Vellasco Júnior, W.T.; Pinto, L.C.; Burbano, R.M.R.; Yoneda, J.D.; Leal, K.Z.; Gomes, C.R.B.; Wardell, J.L.; et al. Synthesis and Biological Evaluation of Novel 6-Hydroxy-benzo[d][1,3]oxathiol-2-one Schiff Bases as Potential Anticancer Agents. Molecules 2015, 20, 1968-1983. https://doi.org/10.3390/molecules20021968
Chazin EDL, Sanches PDS, Lindgren EB, Vellasco Júnior WT, Pinto LC, Burbano RMR, Yoneda JD, Leal KZ, Gomes CRB, Wardell JL, et al. Synthesis and Biological Evaluation of Novel 6-Hydroxy-benzo[d][1,3]oxathiol-2-one Schiff Bases as Potential Anticancer Agents. Molecules. 2015; 20(2):1968-1983. https://doi.org/10.3390/molecules20021968
Chicago/Turabian StyleChazin, Eliza De Lucas, Paola De Souza Sanches, Eric Brazil Lindgren, Walcimar Trindade Vellasco Júnior, Laine Celestino Pinto, Rommel Mario Rodríguez Burbano, Julliane Diniz Yoneda, Kátia Zaccur Leal, Claudia Regina Brandão Gomes, James Lewis Wardell, and et al. 2015. "Synthesis and Biological Evaluation of Novel 6-Hydroxy-benzo[d][1,3]oxathiol-2-one Schiff Bases as Potential Anticancer Agents" Molecules 20, no. 2: 1968-1983. https://doi.org/10.3390/molecules20021968
APA StyleChazin, E. D. L., Sanches, P. D. S., Lindgren, E. B., Vellasco Júnior, W. T., Pinto, L. C., Burbano, R. M. R., Yoneda, J. D., Leal, K. Z., Gomes, C. R. B., Wardell, J. L., Wardell, S. M. S. V., Montenegro, R. C., & Vasconcelos, T. R. A. (2015). Synthesis and Biological Evaluation of Novel 6-Hydroxy-benzo[d][1,3]oxathiol-2-one Schiff Bases as Potential Anticancer Agents. Molecules, 20(2), 1968-1983. https://doi.org/10.3390/molecules20021968