An Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones and Thiones Catalyzed by a Novel Brønsted Acidic Ionic Liquid under Solvent-Free Conditions
Abstract
:1. Introduction

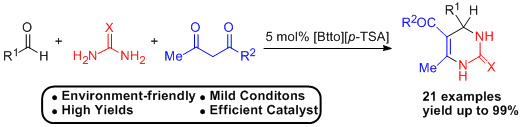
2. Results and Discussion
| Entry | Cat. (mol %) | Temp. (°C) | Time | Yield (%) b |
|---|---|---|---|---|
| 1 | 1 | 90 | 40 min | 69 |
| 2 | 2 | 90 | 40 min | 82 |
| 3 | 3 | 90 | 40 min | 86 |
| 4 | 4 | 90 | 40 min | 80 |
| 5 | 5 | 90 | 40 min | 94 |
| 6 | 6 | 90 | 40 min | 81 |
| 7 | 10 | 90 | 40 min | 91 |
| 8 | 5 | 90 | 10 min | 87 |
| 9 | 5 | 90 | 20 min | 94 |
| 10 | 5 | 90 | 30 min | 96 |
| 11 | 5 | 80 | 30 min | 89 |
| 12 | 5 | 100 | 30 min | 95 |
| 13 | 5 | 30 | 5 h | 63 |
| 14 | 5 | 30 | 10 h | 81 |
| 15 | 5 | 30 | 15 h | 82 |
| Entry | R1 | R2 | X | Yields b (%) | Mp (°C) c | |
|---|---|---|---|---|---|---|
| Found | Found | |||||
| 4a | C6H5 | EtO | O | 96 | 202–204 | 206 [26] |
| 4b | 4-CH3O-C6H4 | EtO | O | 97 | 203–205 | 205–207 [26] |
| 4c | C6H5-CH=CH | EtO | O | 98 | 228–230 | 230–232 [26] |
| 4d | 4-F-C6H4 | EtO | O | 92 | 180–183 | 182–184 [26] |
| 4e | 3-Br-C6H4 | EtO | O | 99 | 183–185 | 185–186 [41] |
| 4f | 4-(CH3)2N-C6H4 | EtO | O | 95 | 250–253 | 253–254 [19] |
| 4g | 3-Cl-C6H4 | EtO | O | 97 | 194–196 | 193–194 [12] |
| 4h | 4-Cl-C6H4 | EtO | O | 93 | 207–210 | 209–212 [26] |
| 4i | 3-O2N-C6H4 | EtO | O | 92 | 225–227 | 227–228 [27] |
| 4j | 3-CH3O-4-HO-C6H3 | EtO | O | 97 | 232–233 | 232–233 [38] |
| 4k | 2- HO-C6H4 | EtO | O | 88 | 200–202 | 199–201 [12] |
| 4l | 3- HO-C6H4 | EtO | O | 98 | 165–167 | 167–170 [26] |
| 4m | 4-HO-C6H4 | EtO | O | 98 | 224–227 | 227–228 [27] |
| 4n | 3,4-(CH3O)2-C6H3 | EtO | O | 96 | 173–175 | 174–176 [27] |
| 4o | 2,4-(Cl)2-C6H3 | EtO | O | 94 | 250–252 | 251–252 [12] |
| 4p | 2-Furyl | EtO | O | 79 | 205–206 | 202–204 [12] |
| 4q | C6H5 | EtO | S | 98 | 205–206 | 207–208 [26] |
| 4r | 4-HO-C6H4 | EtO | S | 96 | 200–202 | 202–203 [26] |
| 4s | 4- (CH3)2N-C6H4 | EtO | S | 89 | 207–209 | 209–210 [26] |
| 4t | C6H5 | MeO | O | 89 | 211–213 | 212–213 [26] |
| 4u | 3-O2N-C6H4 | MeO | O | 99 | 272–275 | 273–275 [26] |
| Entry | R1 | R2 | X | Yields b (%) | Mp (°C) c | |
|---|---|---|---|---|---|---|
| Found | Reported (Lit.) | |||||
| 4a | C6H5 | EtO | O | 77 | 202–205 | 206 [26] |
| 4b | 4-CH3O-C6H4 | EtO | O | 89 | 206–208 | 205–207 [26] |
| 4c | C6H5-CH=CH | EtO | O | 88 | 230–232 | 230–232 [26] |
| 4d | 4-F-C6H4 | EtO | O | 90 | 180–182 | 182–184 [26] |
| 4e | 3-Br-C6H4 | EtO | O | 85 | 182–185 | 185–186 [41] |
| 4g | 3-Cl-C6H4 | EtO | O | 82 | 190–192 | 193–194 [12] |
| 4h | 4-Cl-C6H4 | EtO | O | 85 | 210–212 | 209–212 [26] |
| 4i | 3-O2N-C6H4 | EtO | O | 93 | 225–226 | 227–228 [27] |
| 4j | 3-CH3O-4-HO-C6H3 | EtO | O | 76 | 230–233 | 232–233 [38] |
3. Experimental Section
3.1. General Procedure for the Synthesis of 3,4-Dihydropyrimidine-2(1H)-Ones and Thiones
3.2. General Procedure for the Synthesis of [Btto][p-TSA]

3.3. Physical and Spectroscopic Data of Products
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kappe, C.O. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res. 2000, 33, 879–888. [Google Scholar]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type-a literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Canto, R.F.S.; Bernardi, A.; Battastini, A.M.O.; Russowsky, D.; Eifler-Lim, V.L. Synthesis of Dihydropyrimidin-2-one/thione Library and Cytotoxic Activity against the Human U138-MG and Rat C6 Glioma Cell Lines. J. Braz. Chem. Soc. 2011, 22, 1379–1388. [Google Scholar] [CrossRef]
- Atwal, K.S.; Rovnyak, G.C.; Kimball, S.D.; Floyd, D.M.; Moreland, S.; Swanson, B.N.; Gougoutas, J.Z.; Schwartz, J.; Smillie, K.M.; Malley, M.F. Dihydropyrimidine calcium channel blockers. 2. 3-substituted-4-aryl-1,4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. J. Med. Chem. 1990, 33, 2629–2635. [Google Scholar]
- Atwal, K.S.; Rovnyak, G.C.; O’Reilly, B.C.; Schwartz, J. Substituted 1,4-dihydropyrimidines. 3.1 synthesis of selectively functionalized 2-hetero-1,4-dihydropyrimidines. J. Org. Chem. 1989, 54, 5898–5907. [Google Scholar]
- Heys, L.; Moore, C.G.; Murphy, P.J. The guanidine metabolites of ptilocaulis spiculifer and related compounds; isolation and synthesis. Chem. Soc. Rev. 2000, 29, 57–67. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Burtoloso, A.C.B.; Kossuga, M.H. The chemistry and biology of organic guanidine derivatives. Nat. Prod. Rep. 2008, 25, 919–954. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Kumar, N.V.; Kokke, W.C.; Bean, M.F.; Freyer, A.J.; Brosse, C.D.; Mai, S.; Truneh, A.; Faulkner, D.J.; Carte, B.; et al. Novel alkaloids from the Sponge Batzella sp.: Inhibitors of HIV gp l20-human CD4 binding. J. Org. Chem. 1995, 60, 1182–1188. [Google Scholar]
- Snider, B.B.; Chen, J.; Patil, A.D.; Freyer, A.J. Synthesis of the tricyclic portions of batzelladines A, B and D. Revision of the stereoehemistry of batzelladines A and D. Tetrahedron Lett. 1996, 37, 6977–6980. [Google Scholar] [CrossRef]
- Biginelli, P. Aldureides of ethylic acetoacetate and ethylic oxaloacetate. Gazz. Chim. Ital. 1893, 23, 360–416. [Google Scholar]
- Banik, B.K.; Reddy, A.T.; Datta, A.; Mukhopadhyay, C. Microwave-induced bismuth nitrate-catalyzed synthesis of dihydropyrimidones via Biginelli condensation under solventless conditions. Tetrahedron Lett. 2007, 48, 7392–7394. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S. Titanium dioxide supported on MWCNTs as an eco-friendly catalyst in the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones accelerated under microwave irradiation. New J. Chem. 2014, 38, 3514–3521. [Google Scholar] [CrossRef]
- Li, J.T.; Han, J.F.; Yang, J.H.; Li, T.S. An efficient synthesis of 3,4-dihydropyrimidin-2-ones catalyzed by NH2SO3H under ultrasound irradiation. Ultrason. Sonochem. 2003, 10, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Deng, Y. Ionic liquids catalyzed Biginelli reaction under solvent-free conditions. Tetrahedron Lett. 2001, 42, 5917–5919. [Google Scholar] [CrossRef]
- Zhu, A.; Li, Q.; Li, L.; Wang, J. One-pot synthesis of 3,4-dihydro-2(H)-pyrimidinones catalyzed by reusable acidic choline-based ionic liquids. Catal. Lett. 2013, 143, 463–468. [Google Scholar] [CrossRef]
- Rahman, M.; Sarkar, A.; Ghosh, M.; Majee, A.; Hajra, A. Catalytic application of task Specific ionic liquid on the synthesis of benzoquinazolinone derivatives by a multicomponent reaction. Tetrahedron Lett. 2014, 55, 235–239. [Google Scholar] [CrossRef]
- Ma, Y.; Qian, C.; Wang, L.; Yang, M. Lanthanide triflate catalyzed Biginelli reaction. One-pot synthesis of dihydropyrimidinones under solvent-free conditions. J. Org. Chem. 2000, 65, 3864–3868. [Google Scholar]
- Tu, S.; Fang, F.; Miao, C.; Jiang, H.; Feng, Y.; Shi, D.; Wang, X. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using boric acid as catalyst. Tetrahedron Lett. 2003, 44, 6153–6155. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, G.S. K.K.; Reddy, K.B.; Yadav, J.S. Vanadium(III) chloride catalyzed Biginelli condensation: Solution phase library generation of dihydropyrimidin-(2H)-ones. Tetrahedron Lett. 2003, 44, 6497–6499. [Google Scholar] [CrossRef]
- Su, W.; Li, J.; Zheng, Z.; Shen, Y. One-pot synthesis of dihydropyrimidiones catalyzed by strontium(II) triflate under solvent-free conditions. Tetrahedron Lett. 2005, 46, 6037–6040. [Google Scholar] [CrossRef]
- Debache, A.; Amimour, M.; Belfaitah, A.; Rhouati, S.; Carboni, B. A one-pot Biginelli synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones catalyzed by triphenylphosphine as Lewis base. Tetrahedron Lett. 2008, 49, 6119–6121. [Google Scholar] [CrossRef]
- Fu, N.Y.; Yuan, Y.F.; Pang, M.L.; Wang, J.T.; Peppe, C. Indium(III) halides-catalyzed preparation of ferrocene-dihydropyrimidinones. J. Organomet. Chem. 2003, 672, 52–57. [Google Scholar] [CrossRef]
- Kolvari, E.; Koukabi, N.; Armandpour, O. A simple and efficient Synthesis of 3,4-dihydropyrimidin-2-(1H)-ones via Biginelli reaction catalyzed by nanomagnetic-supported sulfonic acid. Tetrahedron 2014, 70, 1383–1386. [Google Scholar] [CrossRef]
- Starcevich, J.T.; Laughlin, T.J.; Mohan, R.S. Iron(III) tosylate catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via the Biginelli reaction. Tetrahedron Lett. 2013, 54, 983–985. [Google Scholar] [CrossRef]
- Siddiqui, Z.N. Bis[(L)prolinato-N,O] Zn-water: A green catalytic system for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones via the Biginelli reaction. Comptes Rendus Chimie 2013, 16, 183–188. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Aswar, S.A.; Muskawar, P.N.; Bhagat, P.R.; Kumar, S.S. Development and efficient 1-Glycyl-3-methyl imidazolium chloride-copper(II) complex catalyzed highly enantioselective synthesis of 3, 4-dihydropyrimidin-2(1H)-ones. J. Organomet. Chem. 2013, 723, 154–162. [Google Scholar] [CrossRef]
- Azizian, J.; Mohammadi, A.A.; Karimi, A.R.; Mohammadizadeh, M.R. KAl(SO4)2·12H2O supported on silica gel as a novel heterogeneous system catalyzed Biginelli reaction one-pot synthesis of dihydropyrimidinones under solvent-free conditions. Appl. Catal. A Gen. 2006, 300, 85–88. [Google Scholar]
- Ahn, B.J.; Gang, M.S.; Chae, K.; Oh, Y.; Shin, J.; Chang, W. A Microwave-assisted synthesis of 3,4-dihydro-pyrimidin-2-(1H)-ones catalyzed by FeCl3-supported nanopore silica under solvent-free conditions. J. Ind. Eng. Chem. 2008, 14, 401–405. [Google Scholar] [CrossRef]
- Kour, G.; Gupta, M.; Rajnikant, S.P.; Gupta, V.K. SiO2-CuCl2: An efficient and recyclable heterogeneous catalyst for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. J. Mol. Catal. A Chem. 2014, 392, 260–269. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S. MnO2-MWCNT nanocomposites as efficient catalyst in the synthesis of Biginelli-type compounds under microwave radiation. J. Mol. Catal. A Chem. 2013, 373, 72–77. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S. A novel protocol for solvent-free synthesis of 4,6-Diaryl-3,4-Dihydropyrimidine-2(1H)-ones catalyzed by metal oxide—MWCNTs nanocomposites. J. Mol. Struct. 2014, 1074, 71–78. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S. Biginelli reaction on Fe3O4-MWCNT nanocomposite: Excellent reactivity and facile recyclability of the catalyst combined with ultrasound irradiation. RSC Adv. 2013, 3, 17962–17967. [Google Scholar] [CrossRef]
- Zamani, F.; Izadi, E. Synthesis and characterization of sulfonated-phenylacetic acid coated Fe3O3 nanoparticles as a novel acid magnetic catalyst for Biginelli reaction. Catal. Commun. 2013, 42, 104–108. [Google Scholar] [CrossRef]
- Keivanloo, A.; Mirzaee, M.; Bakherad, M.; Soozani, A. Boehmite nanoparticle catalyst for the one-pot multicomponent synthesis of 3,4-dihydropyrimidin-2-(1H)-ones and thiones under solvent-free conditions. Chin. J. Catal. 2014, 35, 362–367. [Google Scholar] [CrossRef]
- Ghomi, J.S.; Teymuri, R.; Ziarati, A. A green synthesis of 3,4-dihydropyrimidine-2(1H)-one/thione derivatives using nanosilica-supported Tin(II) chloride as a heterogeneous nanocatalyst. Monatsh. Chem. 2013, 144, 1865–1870. [Google Scholar] [CrossRef]
- Dhumaskar, K.L.; Meena, S.N.; Ghadi, S.C.; Tilve, S.G. Graphite catalyzed solvent free synthesis of dihydropyrimidin-2(1H)-ones/thiones and their antidiabetic activity. Bioorg. Med. Chem. Lett. 2014, 24, 2897–2899. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-B.; Wang, N.; Wu, W.-X.; Le, Z.-G.; Yu, X.-Q. Trypsin-catalyzed tandem reaction: One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones by in situ formed acetaldehyde. J. Biotechnol. 2014, 170, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Salehi, P.; Dabiri, M.; Zolfigol, M.A.; Fard, M.A.B. Silica sulfuric acid: An efficient and reusable catalyst for the one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2003, 44, 2889–2891. [Google Scholar] [CrossRef]
- Kumar, K.A.; Kasthuraiah, M.; Reddy, C.S.; Reddy, C.D. Mn(OAc)3·2H2O-mediated three-component, one-pot, condensation reaction: An efficient synthesis of 4-aryl-substituted 3,4-dihydropyrimidin-2-ones. Tetrahedron Lett. 2001, 42, 7873–7875. [Google Scholar] [CrossRef]
- Nandurkar, N.S.; Bhanushali, M.J.; Bhor, M.D.; Bhanage, B.M. Y(NO3)3·6H2O: A novel and reusable catalyst for one pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. J. Mol. Catal. A Chem. 2007, 271, 14–17. [Google Scholar] [CrossRef]
- Ghosh, R.; Maiti, S.; Chakraborty, A. In(OTf)3-catalysed one-pot synthesis of 3,4-dihydropyrimidin-2(lH)-ones. J. Mol. Catal. A Chem. 2004, 217, 47–50. [Google Scholar] [CrossRef]
- Ahmed, N.; van Lier, J.E. TaBr5-catalyzed Biginelli reaction: One-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones under solvent-free conditions. Tetrahedron Lett. 2007, 48, 5407–5409. [Google Scholar] [CrossRef]
- Adib, M.; Ghanbary, K.; Mostofi, M.; Ganjali, M.R. Efficient Ce(NO3)3·6H2O-catalyzed solvent-free synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Molecules 2006, 11, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Karade, H.N.; Sathe, M.; Kaushik, M.P. Synthesis of 4-aryl substituted 3,4-dihydropyrimidinones using silica-chloride under solvent free conditions. Molecules 2007, 12, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Qi, D.Y. An efficient and solvent-free one-pot synthesis of dihydropyrimidinones under microwave irradiation. Chin. Chem. Lett. 2007, 18, 647–650. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Z.; Yao, Z.; Xu, F.; Shen, Q. Efficient synthesis of pyrimidinone derivatives by ytterbium chloride catalyzed Biginelli-type reaction under solvent-free conditions. Tetrahedron Lett. 2009, 50, 1622–1624. [Google Scholar] [CrossRef]
- Ahmed, B.; Khan, R.A.; Habibullah; Keshari, M. An improved synthesis of Biginelli-type compounds via phase-transfer catalysis. Tetrahedron Lett. 2009, 50, 2889–2892. [Google Scholar] [CrossRef]
- Alvim, H.G.O.; Lima, T.B.; de Oliveira, A.L.; de Oliveira, H.C.B.; Silva, F.M.; Gozzo, F.C.; Souza, R.Y.; da Silva, W.A.; Neto, B.A.D. Facts, presumptions, and myths on the solvent-free and catalyst-free Biginelli reaction. What is catalysis for? J. Org. Chem. 2014, 79, 3383–3397. [Google Scholar]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Banerjee, S. Ionic liquid as catalyst and reaction medium. The dramatic influence of a task-specific ionic liquid, [bmIm]OH, in michael addition of active methylene compounds to conjugated ketones, carboxylic esters, and nitriles. Org. Lett. 2005, 7, 3049–3052. [Google Scholar]
- Qiao, K.; Hagiwara, H.; Yokoyama, C. Acidic ionic liquid modified silica gel as novel solid catalysts for esterification and nitration reactions. J. Mol. Catal. A Chem. 2006, 246, 65–69. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Li, Y. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-(thio)ones using strontium(II) nitrate as a catalyst. J. Mol. Catal. A Chem. 2006, 258, 367–370. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Liu, C.; Wang, J. An efficient synthesis of 4-substituted pyrazolyl-3,4-dihydropyrimidin-2(1H)-(thio)ones catalyzed by Mg(ClO4)2 under ultrasound irradiation. J. Mol. Catal. A Chem. 2006, 253, 207–211. [Google Scholar] [CrossRef]
- Liu, C.J.; Wang, J.D. Copper(II) sulfamate: An efficient catalyst for the one-pot synthesis of 3,4-dihydropyrimidine-2(1H)-ones and thiones. Molecules 2009, 14, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Wang, J.D. Ultrasound-assisted synthesis of novel 4-(2-phenyl-1,2,3-triazol-4-yl)-3,4-dihydropyrimidin-2(1H)-(Thio)ones catalyzed by Sm(ClO4)3. Molecules 2010, 15, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: All samples are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, B.; Zhang, X.; Huang, J.; Liu, C. An Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones and Thiones Catalyzed by a Novel Brønsted Acidic Ionic Liquid under Solvent-Free Conditions. Molecules 2015, 20, 3811-3820. https://doi.org/10.3390/molecules20033811
Zhang Y, Wang B, Zhang X, Huang J, Liu C. An Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones and Thiones Catalyzed by a Novel Brønsted Acidic Ionic Liquid under Solvent-Free Conditions. Molecules. 2015; 20(3):3811-3820. https://doi.org/10.3390/molecules20033811
Chicago/Turabian StyleZhang, Yonghong, Bin Wang, Xiaomei Zhang, Jianbin Huang, and Chenjiang Liu. 2015. "An Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones and Thiones Catalyzed by a Novel Brønsted Acidic Ionic Liquid under Solvent-Free Conditions" Molecules 20, no. 3: 3811-3820. https://doi.org/10.3390/molecules20033811
APA StyleZhang, Y., Wang, B., Zhang, X., Huang, J., & Liu, C. (2015). An Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones and Thiones Catalyzed by a Novel Brønsted Acidic Ionic Liquid under Solvent-Free Conditions. Molecules, 20(3), 3811-3820. https://doi.org/10.3390/molecules20033811