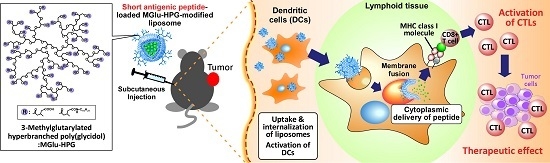
Improvement of Peptide-Based Tumor Immunotherapy Using pH-Sensitive Fusogenic Polymer-Modified Liposomes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Peptide-Loaded Liposomes and Selection of Adjuvant
2.2. Intracellular Distribution of Peptide-Loaded Liposomes
2.3. Induction of Cellular Immune Responses in Vivo
2.4. Induction of Antitumor Responses
2.5. Therapeutic Effects of Peptide-Loaded Liposomes on Tumor-Bearing Mice
3. Materials and Methods
3.1. Materials
3.2. Liposome Preparation
3.3. Cell Culture
3.4. Animals
3.5. Intracellular Behavior of Liposomes
3.6. Cytokine Production from Cells Treated with Liposomes
3.7. CTL Assay
3.8. Induction of Antitumor Immunity by Liposomes
3.9. Therapeutic Effects Induced by Liposomes on Tumor-Bearing Mice
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oka, Y.; Tsuboi, A.; Taguchi, T.; Osaki, T.; Kyo, T.; Nakajima, H.; Elisseeva, O.A.; Oji, Y.; Kawakami, M.; Ikegame, K.; et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc. Natl. Acad. Sci. USA 2004, 101, 13885–13890. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccine. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bade, E.; Kuniyoshi, C.; Spears, L.; Jeffery, G.; Marty, V.; Groshen, S.; Weber, J. Phase I trial of a MART-1 peptide vaccine with incomplete Freund’s adjuvant for resected high-risk melanoma. Clin. Cancer Res. 1999, 5, 2756–2765. [Google Scholar] [PubMed]
- Rosenberg, S.A.; Dudley, M.E. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol. 2009, 21, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Steinman, R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef]
- Robbins, P.F.; Lu, Y.C.; El-Gamil, M.; Li, Y.F.; Gross, C.; Gartner, J.; Lin, J.C.; Teer, J.K.; Cliften, P.; Tycksen, E.; et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 2013, 19, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, S.; Vormehr, M.; van de Roemer, N.; Diken, M.; Löwer, M.; Diekmann, J.; Boegel, S.; Schrörs, B.; Vascotto, F.; Castle, J.C.; et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015, 520, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Komori, H.; Nakatsura, T.; Senju, S.; Yoshitake, Y.; Motomura, Y.; Ikuta, Y.; Fukuma, D.; Yokomine, K.; Harao, M.; Beppu, T.; et al. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin. Cancer Res. 2006, 12, 2689–2697. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, Y.; Zhang, G.; Ke, Y.; Yan, Y.; Wu, L.; Huang, Q.; Zeng, G.; Ying, H.; Jiao, S. Identification of an HLA-DPB1*0501 restricted Melan-A/MART-1 epitope recognized by CD4+ T lymphocytes: Prevalence for immunotherapy in Asian populations. J. Immunother. 2011, 34, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Osen, W.; Soltek, S.; Song, M.; Leuchs, B.; Steitz, J.; Tuting, T.; Eichmuller, S.B.; Nguyen, X.D.; Schadendorf, D.; Paschen, A. Screening of human tumor antigens for CD4 T cell epitopes by combination of HLA-transgenic mice, recombinant adenovirus and antigen peptide libraries. PLoS ONE 2010, 5, e14137. [Google Scholar] [CrossRef] [PubMed]
- Izumoto, S.; Tsuboi, A.; Oka, Y.; Suzuki, T.; Hashiba, T.; Kagawa, N.; Hashimoto, N.; Maruno, M.; Elisseeva, O.A.; Shirakata, T.; et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J. Neurosurg. 2008, 108, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Yoshikawa, T.; Nobuoka, D.; Shirakawa, H.; Kuronuma, T.; Motomura, Y.; Mizuno, S.; Ishii, H.; Nakachi, K.; Konishi, M.; et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: Immunologic evidence and potential for improving overall survival. Clin. Cancer Res. 2012, 18, 3686–3696. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Yoshikawa, T.; Fujii, S.; Mitsunaga, S.; Nobuoka, D.; Mizuno, S.; Takahashi, M.; Yamauchi, C.; Endo, I.; Nakatsura, T. Remarkable tumor lysis in a hepatocellular carcinoma patient immediately following glypican-3-derived peptide vaccination: An autopsy case. Hum. Vaccines Immunother. 2013, 9, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Bijker, M.S.; van den Eeden, S.J.; Franken, K.L.; Melief, C.J.; Offringa, R.; van der Burg, S.H. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J. Immunol. 2007, 179, 5033–5040. [Google Scholar] [CrossRef] [PubMed]
- Hailemichael, Y.; Dai, Z.; Jaffarzad, N.; Ye, Y.; Medina, M.A.; Huang, X.F.; Dorta-Estremera, S.M.; Greeley, N.R.; Nitti, G.; Peng, W.; et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 2013, 19, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Jiang, H.; Wang, T.; Zhang, Z.; Gong, T.; Sun, X. Cationic micelle delivery of Trp2 peptide for efficient lymphatic draining and enhanced cytotoxic T-lymphocyte responses. J. Control. Release 2015, 200, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, M.; Kaushal, S.; McElroy, M.; Zhang, Y.; Ozkan, C.; Bouvet, M.; Kruse, C.; Grotjahn, D.; Ichim, T.; et al. PLGA nanoparticle-mediated delivery of tumor antigenic peptides elicits effective immune responses. Int. J. Nanomed. 2012, 7, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Kojima, C.; Harada, A.; Tana; Watarai, S.; Kono, K. pH–Sensitive fusogenic polymer-modified liposomes as a carrier of antigenic proteins for activation of cellular immunity. Biomaterials 2010, 31, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Kono, K. Carboxylated hyperbranched poly(glycidol)s for preparation of pH-sensitive liposomes. J. Control. Release 2011, 149, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Hebishima, T.; Yuba, E.; Kono, K.; Takeshima, S.; Ito, Y.; Aida, Y. pH-sensitive fusogenic 3-methyl-glutarylated hyperbranched poly(glycidol) (MGlu-HPG)-conjugated liposome induces antigen-specific cellular and humoral immunity. Clin. Vaccine Immunol. 2012, 19, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Watarai, S.; Kono, K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials 2013, 34, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Mata-Haro, V.; Cekic, C.; Martin, M.; Chilton, P.M.; Casella, C.R.; Mitchel, T.C. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 2007, 316, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, Y.; Yuba, E.; Sakaguchi, N.; Koiwai, K.; Harada, A.; Kono, K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials 2014, 35, 8186–8196. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Komatsu, T.; Ishibashi, M.; Udaka, K. Potent CTL induction by a whole cell pertussis vaccine in anti-tumor peptide immunotherapy. Microbiol. Immunol. 2007, 51, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Bozzotti, P.; Mielcarek, N.; Lambert, P.H.; del Giudice, G.; Locht, C.; Siegrist, C.A. Immunogenicity and protective efficacy of neonatal vaccination against Bordetella pertussis in a murine model: Evidence for early control of pertussis. Infect. Immun. 2002, 70, 3521–3528. [Google Scholar] [CrossRef] [PubMed]
- Masuko, K.; Wakita, D.; Togashi, Y.; Kita, T.; Kitamura, H.; Nishimura, T. Artificially synthesized helper/killer-hybrid epitope long peptide (H/K-HELP): Preparation and immunological analysis of vaccine efficacy. Immunol. Lett. 2015, 163, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.W.; Carbone, F.R.; Bevan, M.J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 1988, 54, 777–785. [Google Scholar] [CrossRef]
- Udaka, K. Modification of Helper T Cell-Inducing Polypeptide. US20140341939 A1, 14 November 2014. [Google Scholar]
- Shen, Z.; Reznikoff, G.; Dranoff, G.; Rock, K.L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 1997, 158, 2723–2730. [Google Scholar] [PubMed]
- Sample Availability: Not available.






© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshizaki, Y.; Yuba, E.; Komatsu, T.; Udaka, K.; Harada, A.; Kono, K. Improvement of Peptide-Based Tumor Immunotherapy Using pH-Sensitive Fusogenic Polymer-Modified Liposomes. Molecules 2016, 21, 1284. https://doi.org/10.3390/molecules21101284
Yoshizaki Y, Yuba E, Komatsu T, Udaka K, Harada A, Kono K. Improvement of Peptide-Based Tumor Immunotherapy Using pH-Sensitive Fusogenic Polymer-Modified Liposomes. Molecules. 2016; 21(10):1284. https://doi.org/10.3390/molecules21101284
Chicago/Turabian StyleYoshizaki, Yuta, Eiji Yuba, Toshihiro Komatsu, Keiko Udaka, Atsushi Harada, and Kenji Kono. 2016. "Improvement of Peptide-Based Tumor Immunotherapy Using pH-Sensitive Fusogenic Polymer-Modified Liposomes" Molecules 21, no. 10: 1284. https://doi.org/10.3390/molecules21101284
APA StyleYoshizaki, Y., Yuba, E., Komatsu, T., Udaka, K., Harada, A., & Kono, K. (2016). Improvement of Peptide-Based Tumor Immunotherapy Using pH-Sensitive Fusogenic Polymer-Modified Liposomes. Molecules, 21(10), 1284. https://doi.org/10.3390/molecules21101284