One Pot Single Step Synthesis and Biological Evaluation of Some Novel Bis(1,3,4-thiadiazole) Derivatives as Potential Cytotoxic Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Pharmacology
Biological Screening of the Synthesized Bis-thiadiazoles for Their Cytotoxic Activity
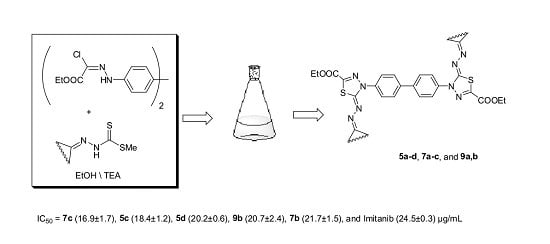
- The bis-thiadiazole derivatives 5c,d, 7b,c and 9b had higher antitumor activity than the standard drug Imatinib.
- The bis-thiadiazole derivatives 5a,b and 9a have moderate activity.
- The bis-thiadiazole derivative 7a has poor antitumor activity against human breast carcinoma cell line (MCF-7).
- The heterocyclic rings such as pyridine in 7c, thiophene in 5c, furan in 5d, 7b and indole in 9b are necessary to have the higher antitumor activity.
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Synthesis of 1,3,4-Thiadiazoline Derivatives 5a–d, 7a–c and 9a,b
3.2. Pharmacology
Anticancer Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, Y.; Li, C.Y.; Wang, X.M.; Yang, Y.H.; Zhu, H.L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Yar, M.S.; Akhter, M.W. Synthesis and anticonvulsant activity of substituted oxadiazole and thiadiazole derivatives. Acta Pol. Pharm. 2009, 66, 393–397. [Google Scholar] [PubMed]
- Azam, F.; Ibn-Rajab, I.A.; Alruiad, A.A. Adenosine A2A receptor antagonists as novel anti-parkinsonian agents: a review of structure-activity relationships. Die Pharm. 2009, 64, 771–775. [Google Scholar] [CrossRef]
- Lalezari, I.; Shafiee, A.; Badaly, A.; Salimi, M.M.; Khoyi, M.A.; Abtahi, F.; Zarrindast, M.R. Synthesis and pharmacological activity of 5-substituted 2-(N,N-dialkylaminoethyl)amino- and 2-N-methyl piperazinyl-1,3,4-thiadiazoles. J. Pharm. Sci. 1975, 64, 1250–1252. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Leonard, J.T.; Roy, K. Exploring QSAR of thiazole and thiadiazole derivatives as potent and selective human adenosine A3 receptor antagonists using FA and GFA techniques. Bioorg. Med. Chem. 2005, 15, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xuan, L.; Zhao, H.; Cheng, J.; Fu, X.; Li, S.; Jing, F.; Liu, Y.; Chen, B. Synthesis and antitumor activities of 1,3,4-thiadiazole derivatives possessing benzisoselenazolone scaffold. Chem. Res. Chin. Univ. 2014, 30, 764–769. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, N.M.; Chang, K.-H.; Shah, K. Synthesis and anticancer activity of 5-(3-indolyl)-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 4664–4668. [Google Scholar] [CrossRef] [PubMed]
- Mathew, V.; Keshavayya, J.; Vaidya, V.P.; Giles, D. Studies on synthesis and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. Eur. J. Med. Chem. 2007, 42, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Noolvi, M.N.; Patel, H.M.; Kamboj, S.; Cameotra, S.S. Synthesis and antimicrobial evaluation of novel 1,3,4-thiadiazole derivatives of 2-(4-formyl-2-methoxyphenoxy) acetic acid. Arab. J Chem. 2012. [Google Scholar] [CrossRef]
- Chawla, A.; Chawla, P.; Baghel, U.S. Synthesis and antimicrobial evaluation of 5-aryl-1,3,4-thiadiazole-2-ylamine derivatives. World J. Pharm. Sci. 2016, 4, 110–114. [Google Scholar]
- Karakus, S.; Rollas, S. Synthesis and antituberculosis activity of new N-phenyl-N′-[4-(5-alkyl/arylamino-1,3,4-thiadiazole-2- yl)phenyl]thioureas. Farmaco 2002, 57, 577–581. [Google Scholar] [CrossRef]
- Oruc, E.E.; Rollas, S.; Kandermirli, F.; Shvets, N.; Dimoglo, A.S. 1,3,4-Thiadiazole derivatives: Synthesis, structure elucidation, and structure-antituberculosis activity relationship investigation. J. Med. Chem. 2004, 47, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Foroumadi, A.; Kargar, Z.; Sakhteman, A.; Sharifzadeh, Z.; Feyzmohammadi, R.; Kazemi, M.; Shafiee, A. Synthesis and antimycobacterial activity of some alkyl [5-(nitroaryl)-1,3,4-thiadiazol-2-ylthio]propionates. Bioorg. Med. Chem. Lett. 2006, 16, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Darwish, E.S.; Kheder, N.A.; Farag, A.M. Synthesis and antimicrobial evaluation of some new pyridine based heterocycles. Heterocycles 2010, 81, 2247–2256. [Google Scholar]
- Farag, A.M.; Kheder, N.A.; Mabkhot, Y.N. Synthesis and antimicrobial evaluation of new pyrazole, thiophene, thiazole and 1,3,4-thiadiazole derivatives incorporating pyrimidine ring. Heterocycles 2009, 78, 1787–1798. [Google Scholar] [CrossRef]
- Kheder, N.A.; Riyadh, S.M.; Asiry, A.M. Azoles and bis-Azoles: Synthesis and biological evaluation as antimicrobial and anti-cancer agents. Chem. Pharm. Bull. 2013, 61, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Badrey, M.G.; Edrees, M.M. Heterocyclisation of 2,5-diacetyl-3,4-disubstituted- thieno[2,3-b]thiophene bis-thiosemicarbazones leading to bis-thiazoles and bis-1,3,4-thiadiazoles as anti-breast cancer agents. J. Chem. Res. 2016, 120–125. [Google Scholar] [CrossRef]
- Dawood, K.M.; Gomha, S.M. Synthesis and anti-cancer activity of 1,3,4-thiadiazole and 1,3-thiazole derivatives having 1,3,4-oxadiazole moiety. J. Heterocycl. Chem. 2015, 52, 1400–1405. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activity of arylazothiazoles and 1,3,4-thiadiazoles using chitosan-grafted-poly(4-vinyl pyridine) as a novel copolymer basic catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Gomha, S.M.; Abdelrazek, F.M.; Abdelrahman, A.H.; Metz, P. Synthesis of some novel thiazole, thiadiazole and 1,4-phenylene-bis-thiazole derivatives. Heterocycles 2016, 92, 954–967. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-aziz, H.M. Synthesis and antitumor activity of 1,3,4-thiadiazole derivatives bearing coumarine ring. Heterocycles 2015, 91, 583–592. [Google Scholar] [CrossRef]
- Gomha, S.M.; Salah, T.A.; Abdelhamid, A.O. Synthesis, characterization and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as potent anticancer agents. Monatsh. Chem. 2015, 146, 149–158. [Google Scholar] [CrossRef]
- Gomha, S.M.; Ahmed, S.A.; Abdelhamid, A.O. Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles, and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin- 5(1H)-one incorporating triazole moiety. Molecules 2015, 20, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Kheder, N.A.; Darwish, E.S. Diethyl 2,2′-[Biphenyl-4,4′-diyldihydrazin-2-yl-1-ylidene]bis (chloroacetate). Molbank 2014, 2014, M813. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Kulandaivelu, U.; Saiko, P.; Graser, G.; Szekeres, T.; Andrei, G.; Snoeck, R.; Balzarini, J.; Jayaprakash, V. Methyl-2-arylidene hydrazinecarbodithioates: Synthesis and biological activity. Chem. Pap. 2013, 67, 650–656. [Google Scholar] [CrossRef]
- Audrieth, L.F.; Scott, E.S.; Kippur, P.S. Hydrazine derivatives of the carbonic and thiocarbonic acids. I. the preparation and properties of thiocarbohydrazide. J. Org. Chem. 1954, 19, 733–741. [Google Scholar] [CrossRef]
- Klayman, D.L.; Bartosevich, J.F.; Griffin, T.S.; Mason, C.J.; Scovill, J.P. 2-Acetylpyridine thiosemicarbazones. 1. A new class of potential antimalarial agents. J. Med. Chem. 1979, 22, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Scovill, J.P.; Klayman, D.L.; Franchino, C.F. 2-Acetylpyridine thiosemicarbazones. 4. Complexes with transition metals as antimalarial and antileukemic agents. J. Med. Chem. 1982, 25, 1261–1264. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Edrees, M.M.; El-Arab, E.E. Synthesis and preliminary in vitro cytotoxic evaluation of some novel bis-heterocycles incorporating thienothiophene. J. Heterocycl. Chem. 2016. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 5a–d, 7a–c and 9a,b are available from the authors.





| Compd. No. | R | IC50 (µg/mL) |
|---|---|---|
| 5a |  | 45.2 ± 1.4 |
| 5b |  | 37.5 ± 1.7 |
| 5c |  | 18.4 ± 1.2 |
| 5d |  | 20.2 ± 0.6 |
| 7a |  | 145.1 ± 3.4 |
| 7b |  | 21.7 ± 1.5 |
| 7c |  | 16.9 ± 1.7 |
| 9a |  | 80.0 ± 2.0 |
| 9b |  | 20.7 ± 2.4 |
| Doxorubicin | ---- | 0.8 ± 0.1 |
| Imatinib | ---- | 24.5 ± 0.3 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomha, S.M.; Kheder, N.A.; Abdelhamid, A.O.; Mabkhot, Y.N. One Pot Single Step Synthesis and Biological Evaluation of Some Novel Bis(1,3,4-thiadiazole) Derivatives as Potential Cytotoxic Agents. Molecules 2016, 21, 1532. https://doi.org/10.3390/molecules21111532
Gomha SM, Kheder NA, Abdelhamid AO, Mabkhot YN. One Pot Single Step Synthesis and Biological Evaluation of Some Novel Bis(1,3,4-thiadiazole) Derivatives as Potential Cytotoxic Agents. Molecules. 2016; 21(11):1532. https://doi.org/10.3390/molecules21111532
Chicago/Turabian StyleGomha, Sobhi M., Nabila A. Kheder, Abdou O. Abdelhamid, and Yahia N. Mabkhot. 2016. "One Pot Single Step Synthesis and Biological Evaluation of Some Novel Bis(1,3,4-thiadiazole) Derivatives as Potential Cytotoxic Agents" Molecules 21, no. 11: 1532. https://doi.org/10.3390/molecules21111532
APA StyleGomha, S. M., Kheder, N. A., Abdelhamid, A. O., & Mabkhot, Y. N. (2016). One Pot Single Step Synthesis and Biological Evaluation of Some Novel Bis(1,3,4-thiadiazole) Derivatives as Potential Cytotoxic Agents. Molecules, 21(11), 1532. https://doi.org/10.3390/molecules21111532