Anticoagulant Activity and Structural Characterization of Polysaccharide from Abalone (Haliotis discus hannai Ino) Gonad
Abstract
:1. Introduction
2. Results and Discussion
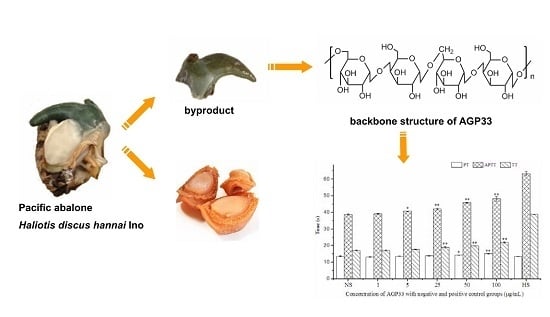
2.1. Preparation and Characterization of Abalone Gonad Polysaccharides
2.2. Partial Acid Hydrolysis of AGP33
2.3. Methylation Analysis of BAGP33
2.4. 13C- and 1H-NMR Analysis of AGP33
2.5. HSQC Analysis of AGP33
2.6. 1H-1H COSY Analysis of AGP33
2.7. Heteronuclear Multiple-Bond Correlation (1H- 13C-HMBC) Analysis of AGP33
2.8. Analysis of the Position of the Sulfate Groups in AGP33
2.9. Anticoagulant Activity
3. Experimental Section
3.1. Materials and Reagents
3.2. General Methods
3.3. Isolation, Purification and Molecular Weight Determination of Abalone Gonad Polysaccharide
3.4. Monosaccharide Composition of AGP33
3.5. Partial Acid Hydrolysis and Purification of Depolymerized AGP33
3.6. Methylation Analysis of BAGP33
3.7. Blood Coagulation Assays
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yuan, X.C.; Zhao, W.W. Bureau of fisheries, ministry of agriculture of china. In China Fishery Statistical Yearbook; China Statistics Press: Beijing, China, 2015; p. 29. [Google Scholar]
- Yang, J.F.; Li, Y.H.; Zhao, J.; Li, P.F.; Zhu, C.; Song, Y.H.; Zhang, L.Y.; Zhu, B.W. Isolation, structural characterization, and lymphopoiesis stimulant activity of a polysaccharide from the abalone gonad. Food Sci. Biotechnol. 2015, 24, 23–30. [Google Scholar] [CrossRef]
- Maas, N.C.; Gracher, A.H.; Sassaki, G.L.; Gorin, P.A.; Lacomini, M.; Cipriani, T.R. Sulfation pattern of citrus pectin and its carboxy-reduced derivatives: Influence on anticoagulant and antithrombotic effects. Carbohydr. Polym. 2012, 9, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Gerbst, A.G.; Ushakova, N.A.; Tsvetkova, E.A.; Dmitrenok, A.S.; Usov, A.I.; Nifantiev, N.E. Anticoagulant and antithrombotic activities of modified xylofucan sulfate from the brown alga Punctaria plantaginea. Carbohydr. Polym. 2016, 136, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Sudharsan, S.; Subhapradha, N.; Seedevi, P.; Shanmugam, V.; Madeswaran, P.; Shanmugam, A.; Srinivasan, A. Antioxidant and anticoagulant activity of sulfated polysaccharide from Gracilaria debilis (Forsskal). Int. J. Biol. Macromol. 2015, 81, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.D.; Pan, R.J.; Deng, X.Y.; Chen, Y.T.; Zhao, G.M.; Wang, C.H. Separation, purification, anticoagulant activity and preliminary structural characterization of two sulfated polysaccharides from seacucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochem. 2014, 49, 1352–1361. [Google Scholar] [CrossRef]
- Olson, S.T.; Björk, I. Mechanism of action of heparin and heparin-like antithrombotics. Perspect. Drug Discov. Des. 1994, 1, 479–501. [Google Scholar] [CrossRef]
- Melo, F.R.; Pereira, M.S.; Foguel, D.; Mourão, P.A. Antithrombin-mediated Anticoagulant Activity of Sulfated Polysaccharides different mechanisms for heparin and sulfated galactans. J. Biol. Chem. 2004, 279, 20824–20835. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.; de Morais, R.M.; Bernardo de Morais, A.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.; de Morais, A.M. Microalgae for the prevention of cardiovascular disease and stroke. Life Sci. 2015, 125, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.; Jia, Z.; Peña, M.J.; Cash, M.; Harper, A.; Blackburn, A.R., II; Darvill, A.; York, W.S. Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydr. Res. 2005, 340, 1826–1840. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, X.Q.; Liu, L.; Yang, T.H.; Li, C.; Fan, H.T.; Jia, M.; Lu, Z.G.; Mei, Q.B. Structure of an antitumor polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr. Polym. 2006, 66, 149–159. [Google Scholar] [CrossRef]
- Uzochukwu, S.; Balogh, E.; Loefler, R.T.; Ngoddy, P.O. Structural analysis by 13C-nuclear magnetic resonance spectroscopy of glucan extracted from natural palm wine. Food Chem. 2002, 76, 287–291. [Google Scholar] [CrossRef]
- Seymour, F.R.; Knapp, R.D.; Bishop, S.H.; Jeanes, A. High temperature enhancement of 13C-NMR chemical-shifts of unusual dextrans, and correlation with methylation structural analysis. Carbohydr. Res. 1979, 68, 123–140. [Google Scholar] [CrossRef]
- Cai, W.R.; Xie, L.L.; Chen, Y.; Zhang, H. Purification, characterization and anticoagulant activity of the polysaccharides from green tea. Carbohydr. Polym. 2013, 92, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Yang, J.H.; Du, Y.M.; Huang, R.H.; Wan, Y.Y.; Li, T. Chemical modification, characterization and structure-anticoagulant activity relationships of Chinese lacquer polysaccharides. Int. J. Biol. Macromol. 2002, 31, 55–62. [Google Scholar] [CrossRef]
- Yang, J.H.; Du, Y.M.; Huang, R.H.; Wan, Y.Y.; Wen, Y. The structure-anticoagulant activity relationships of sulfated lacquer polysaccharide: Effect of carboxyl group and position of sulfation. Int. J. Biol. Macromol. 2005, 36, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Therho, T.T.; Hartiala, K. Method for determination of the sulfate content of glycosamino glycans. Anal. Biochem. 1971, 41, 471–476. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, R.A.; Shashkov, A.S.; Nifant'ev, N.E.; Khatuntseva, E.A.; Usov, A.I. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 20, 108–119. [Google Scholar] [CrossRef]
- Rao, P.; Pattabiraman, T.N. Reevaluation of the phenol-sulphuric acid reaction for the estimation of hexoses and pentoses. Anal. Biochem. 1989, 181, 18–22. [Google Scholar] [CrossRef]
- Rajendra, V.; Ranbir, S.V.; Ahmad, H.W. Separation of aldononitrile acetates of neutral sugars by gas-liquid chromatography and its application to polysaccharides. J. Chromatogr. A 1973, 77, 222–227. [Google Scholar]
- Needs, P.W.; Selvendran, R.R. Avoiding oxidative degradation during sodium hydroxide methyl iodide-mediated Carbohydrate methylation in dimethyl sulfoxide. Carbohydr. Res. 1993, 245, 1–10. [Google Scholar] [CrossRef]
- Yang, J.F.; Zhou, D.Y.; Liang, Z.Y. A new polysaccharide from leaf of Ginkgo biloba L. Fitoterapia 2009, 80, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.







| Linkage Pattern | Methylation Analysis | Chemical Shifts (δ) for the Carbon and Hydrogen Atoms | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Methylated Product | Molar Ratio | C1 | C2 | C3 | C4 | C5 | C6 | ||
| H1 | H2 | H3 | H4 | H5 | H6 | ||||
| A | →3)-α-d-Glcp (1→ | 1,3,5-Ac3-2,4,6-Me3-Glc | 6.7 | 101.02 | 76.54 | 77.69 | 72.48 | 72.23 | 60.53 |
| 5.29 | 3.92 | 4.45 | 3.75 | 4.35 | 3.58, 4.18 | ||||
| B | →4)-α-d-Galp (1→ | 1,4,5-Ac3-2,3,6-Me3-Gal | 13.3 | 99.64 | 76.54 | 77.23 | 81.36 | 72.23 | 60.53 |
| 5.52 | 3.92 | 4.35 | 3.75 | 4.35 | 3.58, 4.18 | ||||
| C | →6)-α-d-Glcp (1→ | 1,5,6-Ac3-2,3,4-Me3-Glc | 6.3 | 100.51 | 76.21 | 77.23 | 72.68 | 72.23 | 69.54 |
| 5.09 | 3.92 | 4.35 | 3.75 | 4.35 | 3.98, 4.45 | ||||
| →6)-α-d-Manp (1→ | 1,5,6-Ac3-2,3,4-Me3-Man | 6.0 | 101.70 | 76.21 | 77.23 | 72.68 | 72.23 | 70.3 | |
| 5.09 | 3.92 | 4.35 | 3.75 | 4.35 | 3.93, 4.18 | ||||
| →6)-α-d-Galp (1→ | 1,5,6-Ac3-2,3,4-Me3-Gal | 3.2 | 101.02 | 76.21 | 77.01 | 72.59 | 72.23 | 68.3 | |
| 5.07 | 3.92 | 4.35 | 3.75 | 4.35 | 4.08, 4.18 | ||||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yang, J.; Song, S.; Zhou, D.; Qiao, W.; Zhu, C.; Liu, S.; Zhu, B. Anticoagulant Activity and Structural Characterization of Polysaccharide from Abalone (Haliotis discus hannai Ino) Gonad. Molecules 2016, 21, 697. https://doi.org/10.3390/molecules21060697
Zhao J, Yang J, Song S, Zhou D, Qiao W, Zhu C, Liu S, Zhu B. Anticoagulant Activity and Structural Characterization of Polysaccharide from Abalone (Haliotis discus hannai Ino) Gonad. Molecules. 2016; 21(6):697. https://doi.org/10.3390/molecules21060697
Chicago/Turabian StyleZhao, Jun, Jingfeng Yang, Shuang Song, Dayong Zhou, Weizhou Qiao, Ce Zhu, Shuyin Liu, and Beiwei Zhu. 2016. "Anticoagulant Activity and Structural Characterization of Polysaccharide from Abalone (Haliotis discus hannai Ino) Gonad" Molecules 21, no. 6: 697. https://doi.org/10.3390/molecules21060697
APA StyleZhao, J., Yang, J., Song, S., Zhou, D., Qiao, W., Zhu, C., Liu, S., & Zhu, B. (2016). Anticoagulant Activity and Structural Characterization of Polysaccharide from Abalone (Haliotis discus hannai Ino) Gonad. Molecules, 21(6), 697. https://doi.org/10.3390/molecules21060697