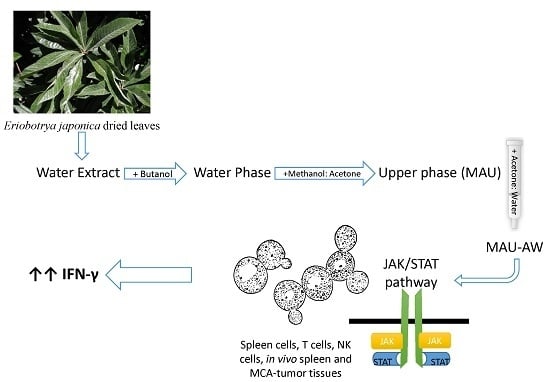
Eriobotrya japonica Water Extract Characterization: An Inducer of Interferon-Gamma Production Mainly by the JAK-STAT Pathway
Abstract
:1. Introduction
2. Results
2.1. Different EJ Extracts Stimulated IFN-γ Production Partially through IL-12 from Mouse Spleen Cells
2.2. MAU-AW Sub-Fraction Stimulated IFN-γ Production Better than the MAU-EW or MAU-ME Sub-Fractions from Unstimulated and Stimulated Mouse Spleen Cells
2.3. MAU-AW Sub-Fraction Induces IFN-γ Production Mainly by JAK-STAT
2.4. EJ Extract and Sub-Fractions Are Not Toxic to Mouse Spleen Cells
2.5. MAU-AW Sub-Fraction Increased the IFN-γ Level in the MCA-Tumor Microenvironment
2.6. MALDI-TOF-MS Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Plant Material Extraction
4.4. Column Chromatography
4.5. Animals
4.6. Mouse Spleen Cells Culture and Cytokines Analysis
4.7. Macrophage Cells Isolation
4.8. NK and T Cells Isolation
4.9. MCA-Induced Tumors, Cell Lines Preparation and Inoculation
4.10. MultiTox-Fluor Multiplex Cytotoxicity Assay
4.11. MALDI-TOF-MS Analysis
4.12. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Lim, T.K. Eriobotrya japonica. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; pp. 381–394. [Google Scholar]
- Cha, D.S.; Eun, J.S.; Jeon, H. Anti-inflammatory and antinociceptive properties of the leaves of Eriobotrya japonica. J. Ethnopharmacol. 2011, 134, 305–312. [Google Scholar] [CrossRef]
- Qa’dan, F.; Petereit, F.; Nahrstedt, A. Prodelphinidin trimers and characterization of a proanthocyanidin oligomer from Cistus albidus. Pharmazie 2003, 58, 416–419. [Google Scholar] [PubMed]
- Afifi, F.; Kasabri, V. Pharmacological and phytochemical appraisal of selected medicinal plants from Jordan with claimed antidiabetic activities. Sci. Pharm. 2013, 81, 889–932. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, N.; De Simone, F.; Pizza, C.; Mahmood, N. Constituents of Eriobotrya japonica. A study of their antiviral properties. J. Nat. Prod. 1992, 55, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, J.; Wang, R.; Wu, Q.; Li, Y.H.; Yu, S.C.; Cheng, W.M.; Wang, Y.Y. Effect of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. leaf on inflammatory cytokine and mediator induction from alveolar macrophages of chronic bronchitis rats. Inflamm. Res. 2007, 56, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, S.L.; Chen, J.C.; Li, C.C.; Lo, H.Y.; Cheng, W.Y.; Lin, J.G.; Chang, Y.H.; Hsiang, C.Y.; Ho, T.Y. Eriobotrya japonica leaf and its triterpenes inhibited lipopolysaccharide-induced cytokines and inducible enzyme production via the nuclear factor-kappaB signaling pathway in lung epithelial cells. Am. J. Chin. Med. 2008, 36, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, T.Y. Anti-inflammatory effect of leaves of Eriobotrya japonica correlating with attenuation of p38 MAPK, ERK, and NF-kappaB activation in mast cells. Toxicol. in Vitro 2009, 23, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.F.; Wang, T.Y.; Zhao, B.; Lv, X.W.; Jin, Y.; Peng, L.; Yu, S.C.; Li, J. Anti-inflammatory effect of triterpenoic acids of Eriobotrya japonica (Thunb.) Lindl. Leaf on rat model of chronic bronchitis. Am. J. Chin. Med. 2009, 37, 309–321. [Google Scholar] [PubMed]
- Shimizu, M.; Fukumura, H.; Tsuji, H.; Tanaami, S.; Hayashi, T.; Morita, N. Anti-inflammatory constituents of topically applied crude drugs. I. Constituents and anti-inflammatory effect of Eriobotrya japonica LINDL. Chem. Pharm. Bull. 1986, 34, 2614–2617. [Google Scholar] [CrossRef] [PubMed]
- Young, H.S.; Chung, H.Y.; Lee, C.K.; Park, K.Y.; Yokozawa, T.; Oura, H. Ursolic acid inhibits aflatoxin B1-induced mutagenicity in a Salmonella assay system. Biol. Pharm. Bull. 1994, 17, 990–992. [Google Scholar] [CrossRef]
- Ito, H.; Kobayashi, E.; Takamatsu, Y.; Li, S.H.; Hatano, T.; Sakagami, H.; Kusama, K.; Satoh, K.; Sugita, D.; Shimura, S.; et al. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem. Pharm. Bull. 2000, 48, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Kobayashi, E.; Li, S.H.; Hatano, T.; Sugita, D.; Kubo, N.; Shimura, S.; Itoh, Y.; Tokuda, H.; Nishino, H.; et al. Antitumor activity of compounds isolated from leaves of Eriobotrya japonica. J. Agric. Food Chem. 2002, 50, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Taguchi, Y.; Akazawa, H.; Nishino, H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol. Pharm. Bull. 2005, 28, 1995–1999. [Google Scholar] [PubMed]
- Matalka, K.Z.; Ali, D.; El Khawad, A.; Qa’dan, F. The differential effect of Eriobotrya japonica hydrophilic leaf extract on cytokines production and modulation. Cytokine 2007, 40, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Alshaker, H.A.; Qinna, N.A.; Qa’dan, F.; Bustami, M.; Matalka, K.Z. Eriobotrya japonica hydrophilic extract modulates cytokines in normal tissues, in the tumor of Meth-A-fibrosarcoma bearing mice, and enhances their survival time. BMC Complement. Altern. Med. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, Y.; Takuma, D.; Onogawa, M.; Yokota, J.; Hamada, A.; Yoshioka, S.; Kusunose, M.; Miyamura, M.; Kyotani, S.; et al. Effect of orally administered Eriobotrya japonica seed extract on allergic contact dermatitis in rats. J. Pharm. Pharmacol. 2007, 59, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Liu, Y.; Zhu, J.; Iguchi, M.; Yoshioka, S.; Miyamura, M.; Kyotani, S. Immunomodulatory effect of Eriobotrya japonica seed extract on allergic dermatitis rats. J. Nutr. Sci. Vitamin (Tokyo) 2010, 56, 145–149. [Google Scholar] [CrossRef]
- Li, E.N.; Luo, J.G.; Kong, L.Y. Qualitative and quantitative determination of seven triterpene acids in Eriobotrya japonica Lindl. by high-performance liquid chromatography with photodiode array detection and mass spectrometry. Phytochem. Anal. 2009, 20, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Hor, M.; Heinrich, M.; Rimpler, H. Proanthocyanidin polymers with antisecretory activity and proanthocyanidin oligomers from Guazuma. ulmifolia bark. Phytochemistry 1996, 42, 109–119. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry 2000, 54, 173–181. [Google Scholar] [CrossRef]
- Mateos-Martín, M.L.; Fuguet, E.; Quero, C.; Pérez-Jiménez, J.; Torres, J.L. New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Rashed, K.N.; Butnariu, M. Isolation and antimicrobial and antioxidant evaluation of bio-active compounds from Eriobotrya japonica stems. Adv. Pharm Bull. 2014, 4, 75–81. [Google Scholar] [PubMed]
- Jung, H.A.; Park, J.C.; Chung, H.Y.; Kim, J.; Choi, J.S. Antioxidant flavonoids and chlorogenic acid from the leaves of Eriobotrya japonica. Arch. Pharm. Res. 1999, 22, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Akazawa, H.; Tabata, K.; Manosroi, A.; Manosroi, J.; Suzuki, T.; Akihisa, T. 3-O-(E)-p-coumaroyl tormentic acid from Eriobotrya japonica leaves induces caspase-dependent apoptotic cell death in human leukemia cell line. Chem. Pharm. Bull. 2011, 59, 378–381. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, N.; De Simone, F.; Aquino, R.; Pizza, C.; Liang, Z.Z. Plant Metabolites. New Sesquiterpene Glycosides from Eriobotrya japonica. J. Nat. Prod. 1990, 53, 810–815. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.L.; Wu, J.L.; Ren, B.R.; Zhang, H.Q. Hypoglycemic effects of a sesquiterpene glycoside isolated from leaves of loquat (Eriobotrya japonica (Thunb.) Lindl. Phytomedicine 2008, 15, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Son, Y.K.; Han, Y.N. Tissue factor inhibitory sesquiterpene glycoside from Eriobotrya japonica. Arch. Pharm. Res. 2004, 27, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Matalka, K.Z.; Alsaadi, M.T.; Qinna, N.A.; Mallah, E.; Awad, R.; Abu Dayyih, W.; Alhussainy, T.; Qadan, F. Enhancing Doxorubicin-induced MCA-fibrosarcoma cytotoxicity by an Eriobotrya japonica hydrophilic butanol-treated extract through natural killer cells activation. J. Cancer Sci. Ther. 2012, S18. [Google Scholar] [CrossRef]
- Schindler, C.; PlumLee, C. Inteferons pen the JAK–STAT pathway. Semin. Cell. Dev. Biol. 2008, 19, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Al-Hanbali, M.; Ali, D.; Bustami, M.; Abdel-Malek, S.; Al-Hanbali, R.; Alhussainy, T.; Qadan, F.; Matalka, K.Z. Epicatechin suppresses IL-6, IL-8 and enhances IL-10 production with NF-κB nuclear translocation in whole blood stimulated system. Neuroendocrinol. Lett. 2009, 30, 131–138. [Google Scholar] [PubMed]
- Mackenzie, G.G.; Carrasquedo, F.; Delfino, J.M.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. FASEB J. 2004, 18, 167–169. [Google Scholar] [PubMed]
- Mackenzie, G.G.; Delfino, M.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Dimeric procyanidins are inhibitors of NF-kappaB-DNA binding. Biochem. Pharmacol. 2009, 78, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, T.; Shishikura, H.; Kitanaka, S.; Toyoshima, S. Effects of psidium components on cytokine productions in helper T cells and type-I allergy. Yakugaku Zasshi 2000, 120, 408–412. [Google Scholar] [PubMed]
- Kato, K.; Yamashita, S.; Kitanaka, S.; Toyoshima, S. Effect of gallic acid derivatives on secretion of Th1 cytokines and Th2 cytokines from anti CD3-stimulated spleen cells. Yakugaku Zasshi 2001, 121, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Matalka, K.Z.; Tutunji, M.F.; Abu-Baker, M.; Abu Baker, Y. Measurement of protein cytokines in tissue extracts by enzyme-linked immunosorbent assays: Application to lipopolysaccharide-induced differential milieu of cytokines. Neuro Endocrinol. Lett. 2005, 26, 231–236. [Google Scholar] [PubMed]
- Wahl, L.M.; Smith, P.D. Current Protocols in Immunology; Coligan, J.E., Bierer, B.E., Margulies, D.H., Shevach, E.M., Strober, W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Sample Availability: Samples of the extracts and subfractions are available from the authors.




| Extract | Concentration | Fold Change in IFN-γ | Fold Change in IL-12 from Unstimulated Macrophages | |||
|---|---|---|---|---|---|---|
| Mouse Spleen Cells | Isolated Mouse Spleen Cells | |||||
| Unstimulated | PHA + LPS Stimulated | Unstimulated T Cells | Unstimulated NK Cells | |||
| WP | 1 μg/mL | 1.4 | 1.5 | 2.3 ** | 0.9 | 1.0 |
| 10 μg/mL | 1.7 ** | 2.7 *** | 2.5 ** | 1.0 | 1.6 | |
| 100 μg/mL | 2.0 ** | 4.2 *** | 4.0 ** | 1.3 | 1.2 | |
| MAU | 1 μg/mL | 1.8 ** | 2.4 ** | 1.7 ** | 1.7 * | 0.8 |
| 10 μg/mL | 2.4 ** | 3.9 *** | 2.5 ** | 1.6 * | 1.4 | |
| 100 μg/mL | 3.2 ** | 5.7 *** | 3.2 ** | 1.0 | 0.7 | |
| MAL | 1 μg/mL | 1.9 * | 4.8 *** | 2.1 * | 0.8 | 1.1 |
| 10 μg/mL | 1.8 * | 5.1 *** | 0.6 | 0.9 | 1.5 | |
| 100 μg/mL | 1.7 | 4.1 ** | 1.2 | 1.8 * | 1.4 | |
| Sub-Fraction | Concentration | Fold Change in IFN-γ | Fold Change in IL-12 | ||
|---|---|---|---|---|---|
| Mouse Spleen Cells | Mouse Spleen Cells | ||||
| Unstimulated | PHA + LPS | Unstimulated | PHA + LPS | ||
| WP | 1 μg/mL | 1.5 | 1.2 | 1.0 | 2.0 |
| 10 μg/mL | 2.4 * | 1.9 * | 1.7 | 2.2 * | |
| MAU-EW | 1 μg/mL | 2.5 * | 3.2 *** | 1.5 | 2.7 ** |
| 10 μg/mL | 2.7 * | 3.3 *** | 1.8 | 2.7 ** | |
| MAU-ME | 1 μg/mL | 3.8 * | 2.5 * | 1.4 | 2.4 * |
| 10 μg/mL | 3.8 * | 2.5 * | 1.5 | 2.0 | |
| MAU-AW | 1 μg/mL | 5.0 ** | 3.1 * | 1.4 | 2.2 |
| 10 μg/mL | 5.4 ** | 3.2 * | 1.8** | 2.4 | |
| Mass (m/z) | Eriobotrya japonica Hydrophilic Extracts and Sub Fractions | References | ||||
|---|---|---|---|---|---|---|
| WE | WP | MAU-EW | MAU-ME | MAU-AW | ||
| 503 ± 2 | Eriojaposide A | Eriojaposide A | [12] | |||
| 507 ± 2 | Ursolic acid derivative | [5,19] | ||||
| 518 ± 2 | 9-O-apiosyl (1–6) glucoside | [12,13] | ||||
| 576 ± 2 | A-type dimeric procyanidin (+proton) | [12,20,21,22] | ||||
| 580 ± 2 | Naringenin-8-C rhamnoglucoside | Naringenin-8-C rhamnoglucoside | Naringenin-8-C rhamnoglucoside | Naringenin-8-C rhamnoglucoside | Naringenin-8-C rhamnoglucoside | [12,23] |
| 588 ± 2 | A-type dimeric procyanidin + (proton) | [12,20,21,22] | ||||
| 598 ± 2 | Quercetin 3- sambubioside | Quercetin 3- sambubioside | Quercetin 3- sambubioside | Quercetin 3- sambubioside | Quercetin 3- sambubioside | [23,24] |
| 610 ± 2 | Kaempferol 3-O-sophoroside | [12,23] | ||||
| 637 ± 2 | Cinchonain glucoside + (sodium) | Cinchonain glucoside + (sodium) | Cinchonain glucoside + (sodium) | Cinchonain glucoside + (sodium) | Cinchonain glucoside + (sodium) | [12] |
| 651 ± 2 | 3-O-coumaroyl tormentic acid | 3-O-coumaroyl tormentic acid | [25] | |||
| 676 ± 2 | Nerolidol 3-O-rhamnopyranosyl glycopyranosides | [26,27,28] | ||||
| 757 ± 2 | Kaempferol 3-O-rhamnosyl glucoside-7-O-rhaminoside | Kaempferol 3-O-rhamnosyl glucoside-7-O-rhaminoside | [23,24] | |||
| 774 ± 2 | Quercetin 3-O-glucosyl-rhamnosyl-glucoside | Quercetin 3-O-glucosyl-rhamnosyl-glucoside | Quercetin 3-O-glucosyl-rhamnosyl-glucoside | Quercetin 3-O-glucosyl-rhamnosyl-glucoside | [23,24] | |
| 867 ± 2 | Procyanidin C-1 + (proton) | Procyanidin C-1 + (proton) | [12,20] | |||
| 1029 ± 2 | Trimeric procyanidin + (gallic acid + glucose + sodium) | Trimeric procyanidin + (gallic acid + glucose + sodium) | [12,20] | |||
| 1158 ± 2 | B-type tetrameric procyanidin or A-type type + (proton) | [12,20] | ||||
| 1175 ±2 | A-type tetrameric procyanidin + (sodium) | [12,20] | ||||
| 1197 ± 2 | B-type tetrameric procyanidin + (sodium) Or dimeric cinchonain including two catechin units | [12,20] | ||||
| 1354 ± 2 | B-type tetrameric procyanidin or A-type type + (sodium + gallic or glucose) | B-type tetrameric procyanidin or A-type type + (sodium + gallic or glucose) | [12,20,21,22] | |||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matalka, K.Z.; Abdulridha, N.A.; Badr, M.M.; Mansoor, K.; Qinna, N.A.; Qadan, F. Eriobotrya japonica Water Extract Characterization: An Inducer of Interferon-Gamma Production Mainly by the JAK-STAT Pathway. Molecules 2016, 21, 722. https://doi.org/10.3390/molecules21060722
Matalka KZ, Abdulridha NA, Badr MM, Mansoor K, Qinna NA, Qadan F. Eriobotrya japonica Water Extract Characterization: An Inducer of Interferon-Gamma Production Mainly by the JAK-STAT Pathway. Molecules. 2016; 21(6):722. https://doi.org/10.3390/molecules21060722
Chicago/Turabian StyleMatalka, Khalid Z., Nada A. Abdulridha, Mujtaba M. Badr, Kenza Mansoor, Nidal A. Qinna, and Fadi Qadan. 2016. "Eriobotrya japonica Water Extract Characterization: An Inducer of Interferon-Gamma Production Mainly by the JAK-STAT Pathway" Molecules 21, no. 6: 722. https://doi.org/10.3390/molecules21060722
APA StyleMatalka, K. Z., Abdulridha, N. A., Badr, M. M., Mansoor, K., Qinna, N. A., & Qadan, F. (2016). Eriobotrya japonica Water Extract Characterization: An Inducer of Interferon-Gamma Production Mainly by the JAK-STAT Pathway. Molecules, 21(6), 722. https://doi.org/10.3390/molecules21060722