Enhanced Glucose Uptake in Human Liver Cells and Inhibition of Carbohydrate Hydrolyzing Enzymes by Nordic Berry Extracts
Abstract
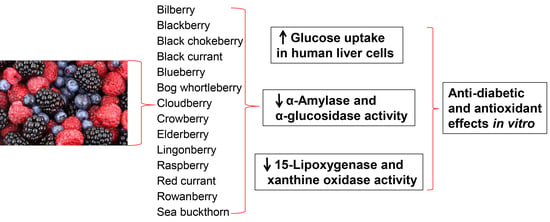
:1. Introduction
2. Results and Discussion
2.1. Total Phenol Content and Phenolic Profile of Extracts
2.2. Glucose Uptake in HepG2-Cells
2.3. Inhibition of α-Amylase and α-Glucosidase
2.4. 15-Lipoxygenase and Xanthine Oxidase Inhibition
3. Materials and Methods
3.1. Berries
3.2. Chemicals
3.3. Pressurized Solvent Extraction (ASE)
3.4. Solid Phase Extraction (SPE) of Berry Extracts
3.5. NMR Spectroscopy
3.6. Total Phenol Content
3.7. 15-Lipoxygenase (15-LO) Inhibition
3.8. Xanthine Oxidase (XO) Inhibition
3.9. α-Glucosidase Inhibition
3.10. α-Amylase Inhibition
3.11. Culturing of HepG2-Cells
3.12. Glucose Uptake in HepG2-Cells
3.13. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 15-LO | 15-lipoxygenase |
| 22-SHC | 22-S-hydroxycholesterol |
| ASE | accelerated solvent extraction |
| DCM | Dichloromethane |
| DMSO | Dimethyl sulfoxide |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EtOH | Ethanol |
| MeOH | Methanol |
| NMR | Nuclear magnetic resonance |
| ROS | Reactive oxygen species |
| SPE | Solid phase extraction |
| T2D | Type 2 diabetes |
| TFA | Trifluoroacetic acid |
| TMS | Tetramethylsilane |
| XO | Xanthine oxidase |
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.; Schwab, U.; Kolehmainen, M.; Paananen, J.; Nygren, H.; Seppänen-Laakso, T.; Poutanen, K.; Hyötyläinen, T.; Risérus, U.; Savolainen, M.J. A healthy Nordic diet alters the plasma lipidomic profile in adults with features of metabolic syndrome in a multicenter randomized dietary intervention. J. Nutr. 2016, 146, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, N.; Rissanen, H.; Knekt, P.; Havulinna, A.; Eriksson, J.; Männistö, S. The healthy Nordic diet and incidence of type 2 diabetes-10-year follow-up. Diabetes Res. Clin. Pract. 2014, 106, e34–e37. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Virtanen, J.K.; Tuomainen, T.-P.; Nurmi, T.; Voutilainen, S. Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2014, 99, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Cherrington, A.D. Control of glucose uptake and release by the liver in vivo. Diabetes 1999, 48, 1198. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Houghton, P.; Soumyanath, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Current developments on the inhibitory effects of berry polyphenols on digestive enzymes. Biofactors 2008, 34, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic cardiovascular disease induced by oxidative stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef] [PubMed]
- Dobrian, A.D.; Lieb, D.C.; Cole, B.K.; Taylor-Fishwick, D.A.; Chakrabarti, S.K.; Nadler, J.L. Functional and pathological roles of the 12-and 15-lipoxygenases. Prog. Lipid Res. 2011, 50, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Boeing, J.S.; Barizão, É.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. A thorough study of reactivity of various compound classes towards the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Doskocz, M.; Jodlowska, E.; Zacharzewska, A.; Lakomiec, J.; Czaja, K.; Kujawski, J. NMR techniques in metabolomic studies: A quick overview on examples of utilization. Appl. Magn. Reson. 2017, 48, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Szajdek, A.; Borowska, E.J. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008, 63, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, M. Antioxidant activity and antimicrobial effect of berry phenolics—A Finnish perspective. Mol. Nutr. Food Res. 2007, 51, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Ø.; Jordheim, M. Chemistry of flavonoid-based colors in plants. In Comprehensive Natural Products II: Chemistry and Biology; Mander, L., Lieu, H.-W.B., Eds.; Elsevier Science: Burlington, NJ, USA, 2010; Volume 1, pp. 547–614. ISBN 978-0-08-045381-1. [Google Scholar]
- Tian, Y.; Liimatainen, J.; Alanne, A.-L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Lätti, A.K.; Riihinen, K.R.; Kainulainen, P.S. Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland. J. Agric. Food Chem. 2008, 56, 190–196. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Jordheim, M.; Enerstvedt, K.H.; Andersen, O.M. Identification of cyanidin 3-O-b-(6′′-(3-hydroxy-3-methylglutaroyl)glucoside) and other anthocyanins from wild and cultivated blackberries. J. Agric. Food Chem. 2011, 59, 7436–7440. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Medica 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; González-Paramás, A.M.; Törrönen, A.R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Kader, F.; Rovel, B.; Girardin, M.; Metche, M. Fractionation and identification of the phenolic compounds of Highbush blueberries (Vaccinium corymbosum, L.). Food Chem. 1996, 55, 35–40. [Google Scholar] [CrossRef]
- Colak, N.; Torun, H.; Gruz, J.; Strnad, M.; Hermosin-Gutiérrez, I.; Hayirlioglu-Ayaz, S.; Ayaz, F.A. Bog bilberry phenolics, antioxidant capacity and nutrient profile. Food Chem. 2016, 201, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Ø. Anthocyanins in fruits of Vaccinium uliginosum L. (Bog Whortleberry). J. Food Sci. 1987, 52, 665–666. [Google Scholar] [CrossRef]
- Kähkönen, M.; Kylli, P.; Ollilainen, V.; Salminen, J.P.; Heinonen, M. Antioxidant activity of isolated ellagitannins from red raspberries and cloudberries. J. Agric. Food Chem. 2012, 60, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Pyysalo, T.; Honkanen, E. The influence of heat on the aroma of cloudberries (Rubus chamaemorus L.). Z. Lebensm. Unters. Forsch. 1977, 163, 25–30. [Google Scholar]
- Ogawa, K.; Sakakibara, H.; Iwata, R.; Ishii, T.; Sato, T.; Goda, T.; Shimoi, K.; Kumazawa, S. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008, 56, 4457–4462. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Heimhuber, B.; Wray, V.; Galensa, R.; Herrmann, K. Benzoylglucoses from two Vaccinium species. Phytochemistry 1990, 29, 2726–2727. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Wyzgoski, F.J.; Paudel, L.; Rinaldi, P.L.; Reese, R.N.; Ozgen, M.; Tulio, A.Z., Jr.; Miller, A.R.; Scheerens, J.C.; Hardy, J.K. Modeling relationships among active components in black raspberry (Rubus occidentalis L.) fruit extracts using high-resolution 1H nuclear magnetic resonance (NMR) spectroscopy and multivariate statistical analysis. J. Agric. Food Chem. 2010, 58, 3407–3414. [Google Scholar] [CrossRef] [PubMed]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; McDougall, G.; Stewart, D.; Heinonen, M. Rowanberry phenolics: Compositional analysis and bioactivities. J. Agric. Food Chem. 2010, 58, 11985–11992. [Google Scholar] [CrossRef] [PubMed]
- Rösch, D.; Bergmann, M.; Knorr, D.; Kroh, L.W. Structure-antioxidant efficiency relationships of phenolic compounds and their contribution to the antioxidant activity of sea buckthorn juice. J. Agric. Food Chem. 2003, 51, 4233–4239. [Google Scholar] [CrossRef] [PubMed]
- Jordheim, M. Isolation, Identification and Properties of Pyranoanthocyanins and Anthocyanin Forms. Ph.D. Thesis, University of Bergen, Bergen, Norway, 22 June 2007. [Google Scholar]
- Cabrita, L. Analysis and Stability of Anthocyanins. Ph.D. thesis, University of Bergen, Bergen, Norway, 1999. [Google Scholar]
- Hilbert, G.; Temsamani, H.; Bordenave, L.; Pedrot, E.; Chaher, N.; Cluzet, S.; Delaunay, J.-C.; Ollat, N.; Delrot, S.; Merillon, J.-M.; et al. Flavonol profiles in berries of wild Vitis accessions using liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectrometry. Food Chem. 2015, 169, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.F.; Kuczkowiak, U.; Nahrstedt, A. Solvent effects in the structure dereplication of caffeoyl quinic acids. Magn. Reson. Chem. 1999, 37, 827–836. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, H.-K. Sanguiin H-6 blocks endothelial cell growth through inhibition of VEGF binding to VEGF receptor. Arch. Pharm. Res. 2005, 28, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Phenolic elderberry extracts, anthocyanins, procyanidins and metabolites influence glucose and fatty acid uptake in human skeletal muscle cells. J. Agric. Food Chem. 2017, 65, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Effect of phenolic compounds from elderflowers on glucose- and fatty acid uptake in human myotubes and HepG2-cells. Molecules 2017, 22, 90. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziolkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry polyphenols inhibit α-amylase in vitro: Identifying active components in rowanberry and raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.E.; Slimestad, R.; Barsett, H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Lilley, T.H.; Haslam, E. Polyphenol interactions: Astringency and the loss of astringency in ripening fruit. Phytochemistry 1987, 26, 2937–2942. [Google Scholar] [CrossRef]
- Schenkels, L.C.; Veerman, E.C.; Nieuw Amerongen, A.V. Biochemical composition of human saliva in relation to other mucosal fluids. Crit. Rev. Oral Biol. Med. 1995, 6, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol Chem. 1981, 256, 4494–4497. [Google Scholar] [PubMed]
- Sadeghian, H.; Jabbari, A. 15-Lipoxygenase inhibitors: A patent review. Expert Opin. Ther. Pat. 2016, 26, 65–88. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: Structure–activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Berghe, D.V. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Wangensteen, H.; Samuelsen, A.B.; Malterud, K.E. Antioxidant activity in extracts from coriander. Food Chem. 2004, 88, 293–297. [Google Scholar] [CrossRef]
- Pham, A.T.; Malterud, K.E.; Paulsen, B.S.; Diallo, D.; Wangensteen, H. DPPH radical scavenging and xanthine oxidase inhibitory activity of Terminalia macroptera leaves. Nat. Prod. Commun. 2011, 6, 1125–1128. [Google Scholar] [PubMed]
- Wensaas, A.; Rustan, A.; Lövstedt, K.; Kull, B.; Wikström, S.; Drevon, C.; Hallen, S. Cell-based multiwell assays for the detection of substrate accumulation and oxidation. J. Lipid Res. 2007, 48, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A. Berry anthocyanin intake and cardiovascular health. Mol. Asp. Med. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the extracts are available from the authors. |

| Berries | Total Phenolic Content | ||
|---|---|---|---|
| Berries a | Methanol ASE Extract b | Methanol SPE Extract b | |
| Bilberry | 533 ± 28 | 56 ± 3 | 299 ± 5 |
| Blackberry | 511 ± 32 | 48 ± 3 | 312 ± 9 |
| Black chokeberry | 835 ± 23 | 64 ± 2 | 384 ± 17 |
| Black currant | 413 ± 26 | 50 ± 3 | 306 ± 16 |
| Blueberry | 211 ± 25 | 18 ± 2 | 218 ± 3 |
| Bog whortleberry | 595 ± 37 | 73 ± 5 | 395 ± 19 |
| Cloudberry | 311 ± 13 | 37 ± 2 | 414 ± 14 |
| Crowberry | 630 ± 24 | 85 ± 3 | 467 ± 24 |
| Elderberry | 251 ± 27 | 53 ± 6 | 262 ± 7 |
| Lingonberry | 490 ± 15 | 38 ± 1 | 233 ± 5 |
| Raspberry | 201 ± 12 | 26 ± 2 | 342 ± 17 |
| Red currant | 105 ± 8 | 10 ± 1 | 244 ± 2 |
| Rowanberry | 347 ± 11 | 20 ± 1 | 150 ± 6 |
| Sea buckthorn | 95 ± 3 | 11 ± 0.3 | 89 ± 4 |
| Berries | Anthocyanin (Anthocyanidin) | Flavonol (Glycoside) | Chlorogenic Acids | Ellagi-Tannins | Other | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cy a | Dp a | Mv a | Que a | Myr a | Isorha a | CA a | NCA a | ||||
| Bilberry | + | + | + | [22,23] | |||||||
| Blackberry | + | [24,25] | |||||||||
| Black chokeberry | + | + | + | [19,21,26] | |||||||
| Black currant | + | + | [19,21,27] | ||||||||
| Blueberry | + | + | + | [21,27,28] | |||||||
| Bog whortleberry | + | + | + | + | [27,29,30] | ||||||
| Cloudberry | + | Benzoic acid | [31,32] | ||||||||
| Crowberry | + | + | + | [21,27,33] | |||||||
| Elderberry | + | + | + | [19,27,34] | |||||||
| Lingonberry (cowberry) | 1-O-benzoy-glucose, 6-O-benzoyl-glucose | [21,27,35] | |||||||||
| Raspberry | + | + | [31,36,37] | ||||||||
| Red currant | tr b | Glycosylated phenolic acids | [19,21] | ||||||||
| Rowanberries | + | + | [21,38] | ||||||||
| Sea buckthorn | + | [21,27,39] | |||||||||
| Berries | α-Amylase | α-Glucosidase | ||
|---|---|---|---|---|
| ASE Extract | SPE Extract | ASE Extract | SPE Extract | |
| Bilberry | 12.2 ± 3.1 | 6.2 ± 0.9 | 18.2 ± 0.8 | 9.6 ± 1.0 |
| Blackberry | 13.7 ± 0.9 | 10.1 ± 0.8 | 16.0 ± 1.1 | 9.5 ± 0.7 |
| Black chokeberry | 10.6 ± 1.5 | 6.0 ± 1.0 | 12.0 ± 1.7 | 8.1 ± 0.8 |
| Black currant | 13.5 ± 1.2 | 10.3 ± 2.4 | 24.0 ± 3.2 | 17.0 ± 2.3 |
| Blueberry | 16.1 ± 1.8 | 16.6 ± 2.0 | 25.2 ± 2.9 | 21.3 ± 1.4 |
| Bog whortleberry | 9.1 ± 3.5 | 8.2 ± 1.3 | 18.2 ± 2.7 | 10.5 ± 1.6 |
| Cloudberry | 9.6 ± 0.7 | 6.9 ± 0.8 | 12.6 ± 1.3 | 7.8 ± 0.4 |
| Crowberry | 6.3 ± 0.8 | 5.3 ± 0.9 | 10.9 ± 1.1 | 8.3 ± 0.4 |
| Elderberry | 10.7 ± 0.8 | 7.1 ± 0.8 | 13.5 ± 2.0 | 8.2 ± 0.9 |
| Lingonberry | 17.1 ± 1.2 | 12.0 ± 1.5 | 20.7 ± 3.0 | 17.4 ± 3.3 |
| Raspberry | 12.1 ± 1.0 | 10.2 ± 1.4 | 15.0 ± 0.5 | 8.4 ± 1.2 |
| Red currant | 20.7 ± 3.0 | 17.3 ± 0.9 | 31.6 ± 3.2 | 17.4 ± 0.7 |
| Rowanberry | 11.3 ± 0.5 | 7.5 ± 1.3 | 13.7 ± 1.8 | 10.0 ± 0.3 |
| Sea buckthorn | 21.4 ± 3.2 | 17.2 ± 1.2 | 34.7 ± 3.6 | 17.3 ± 5.6 |
| Acarbose (positive control) | 73.3 ± 4.3 | 84.7 ± 3.8 | ||
| Berries | 15-Lipoxygenase | Xanthine Oxidase |
|---|---|---|
| Bilberry | 69.5 ± 3.1 | 122.0 ± 5.1 |
| Blackberry | 74.8 ± 7.4 | 96.4 ± 2.4 |
| Black chokeberry | 77.5 ± 7.3 | 125.0 ± 4.3 |
| Black currant | 52.2 ± 3.8 | 80.0 ± 14.3 |
| Blueberry | 104.1 ± 3.9 | 73.4 ± 2.5 |
| Bog whortleberry | 63.1 ± 3.6 | 50.4 ± 1.4 |
| Cloudberry | 50.9 ± 2.1 | 101.0 ± 2.1 |
| Crowberry | 46.6 ± 1.7 | 76.0 ± 3.7 |
| Elderberry | 100.3 ± 5.4 | >167 |
| Lingonberry | 77.0 ± 3.6 | 67.2 ± 1.7 |
| Raspberry | 53.5 ± 2.5 | 126 ±7.4 |
| Red currant | 61.6 ± 2.8 | >167 |
| Rowanberry | >167 | >167 |
| Sea buckthorn | >167 | >167 |
| Quercetin (positive control) | 28.1 ± 0.5 | 0.7 ± 0.2 |
| English Name | Scientific Name | Wild or Name of Cultivar | Genus | Origin |
|---|---|---|---|---|
| Bilberry | Vaccinium myrtillus L. | Wild | Ericaceae | Mountain district a, Norway |
| Blackberry | Rubus fruticosus | Wild | Rosaceae | Oslo district b, Norway |
| Black chokeberry | Aronia melanocarpa (Michx.) Elliott | Cultivar Moskva | Rosaceae | Oslo district b, Norway |
| Black currant | Ribes nigrum L. | Cultivar Ben Tron | Grossulariaceae | Oslo district b, Norway |
| Blueberry | Vaccinium corymbosum L. | Cultivar Royal Blue | Ericaceae | Purchased c, Marocco |
| Bog whortleberry | Vaccinium uliginosum L. | Wild | Ericaceae | Mountain district, Norway |
| Cloudberry | Rubus chamaemorus L. | Wild | Rosaceae | Mountain district, Norway |
| Crowberry | Empetrum nigrum L. | Wild | Empetraceae | Mountain district, Norway |
| Elderberry | Sambucus nigra L. | Cultivar Sampo | Adoxaceae | Vestlandet d, Norway |
| Lingonberry (cowberry) | Vaccinium vitis-idaea L. | Wild | Ericaceae | Purchased e, Sweden |
| Raspberry | Rubus idaeus L. | Wild | Rosaceae | Oslo district, Norway |
| Red currant | Ribes rubrum L. | Cultivar Red Dutch | Grossulariaceae | Oslo district, Norway |
| Rowanberry | Sorbus aucuparia L. | Wild | Rosaceae | Oslo district, Norway |
| Sea buckthorn | Elaeagnus rhamnoides L. A. Nelson | Unknown cultivar | Elaeagnaceae | Oslo district, Norway |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, G.T.T.; Nguyen, T.K.Y.; Kase, E.T.; Tadesse, M.; Barsett, H.; Wangensteen, H. Enhanced Glucose Uptake in Human Liver Cells and Inhibition of Carbohydrate Hydrolyzing Enzymes by Nordic Berry Extracts. Molecules 2017, 22, 1806. https://doi.org/10.3390/molecules22101806
Ho GTT, Nguyen TKY, Kase ET, Tadesse M, Barsett H, Wangensteen H. Enhanced Glucose Uptake in Human Liver Cells and Inhibition of Carbohydrate Hydrolyzing Enzymes by Nordic Berry Extracts. Molecules. 2017; 22(10):1806. https://doi.org/10.3390/molecules22101806
Chicago/Turabian StyleHo, Giang Thanh Thi, Thi Kim Yen Nguyen, Eili Tranheim Kase, Margey Tadesse, Hilde Barsett, and Helle Wangensteen. 2017. "Enhanced Glucose Uptake in Human Liver Cells and Inhibition of Carbohydrate Hydrolyzing Enzymes by Nordic Berry Extracts" Molecules 22, no. 10: 1806. https://doi.org/10.3390/molecules22101806
APA StyleHo, G. T. T., Nguyen, T. K. Y., Kase, E. T., Tadesse, M., Barsett, H., & Wangensteen, H. (2017). Enhanced Glucose Uptake in Human Liver Cells and Inhibition of Carbohydrate Hydrolyzing Enzymes by Nordic Berry Extracts. Molecules, 22(10), 1806. https://doi.org/10.3390/molecules22101806