Outlook on the Application of Metal-Liganded Bioactives for Stimuli-Responsive Release
Abstract
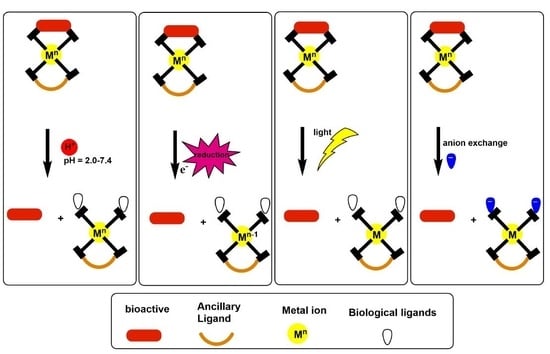
:1. Introduction
2. pH-Triggered Bioactive Release
2.1. Bioinspired Materials
2.2. Self-Assembled Metal-Liganded Bioactives
3. Light-Triggered Bioactive Release
Ruthenium Cage Complexes
4. Redox-Triggered Bioactive Release
5. Ion Activation-Triggered Release of Bioactive
6. Conclusions
- (a)
- In vitro studies must include metal-liganded bioactive release in a physiologically-simulated milieu to fully account for the stability, release mechanism, and possible free metal ion interactions.
- (b)
- Possible inclusion of additional formulation science to overcome challenges faced in the physiological milieu (e.g., enteric targeting for oral systems).
- (c)
- Evaluating whether the drug release kinetics observed translates to in vivo efficiency.
- (d)
- Inclusion of in-depth in vitro cytotoxicity analyses in applicable cell lines and in vivo studies to mitigate the perceived risks involved in using metal carriers.
Acknowledgments
Conflicts of Interest
References
- Ejima, H.; Richardson, J.J.; Caruso, F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today 2017, 12, 136–148. [Google Scholar] [CrossRef]
- Rojas, S.; Devic, T.; Horcajada, P. Metal organic frameworks based on bioactive components. J. Mater. Chem. B 2017, 5, 2560–2573. [Google Scholar] [CrossRef]
- Grindy, S.C.; Holten-Andersen, N. Bio-inspired metal-coordinate hydrogels with programmable viscoelastic material functions controlled by longwave UV light. Soft Matter 2017, 13, 4057–4065. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhou, B.; He, Y.; Pei, Y.; Li, B.; Li, J. Tailoring stimuli-responsive delivery system driven by metal–ligand coordination bonding. Int. J. Nanomed. 2017, 12, 3315–3330. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Tong, R.; Wang, Z.; Guo, S.; Xia, H. High intensity focused ultrasound responsive metallo-supramolecular block copolymer micelles. Langmuir 2014, 30, 9524–9532. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Li, J.; He, Y.; Xu, W.; Liu, S.; Li, Y.; Chen, Y.; Li, B. Engineering multifunctional films based on metal-phenolic networks for rational pH-responsive delivery and cell imaging. ACS Biomater. Sci. Eng. 2016, 2, 317–325. [Google Scholar] [CrossRef]
- Chen, W.-H.; Yu, X.; Cecconello, A.; Sohn, Y.S.; Nechushtai, R.; Willner, I. Stimuli-responsive nucleic acid-functionalized metal-organic framework nanoparticles using pH- and metal-ion-dependent DNAzymes as locks. Chem. Sci. 2017, 8, 5769–5780. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, A.K. Transition metal complexes with bioactive ligands: Mechanisms for selective ligand release and applications for drug delivery. Metallomics 2014, 6, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013, 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Piccariello, T. Metal Coordinated Compositions. U.S. Patent 989440 B2, 2 August 2011. [Google Scholar]
- Siddappa, K.; Mane, S.B.; Manikprabhu, D. Spectral characterization and 3D molecular modeling studies of metal complexes involving the O, N-donor environment of quinazoline-4(3H)-one schiff base and their biological studies. Sci. World J. 2014, 2014, 817365. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.V.; Dwivedi, R.; Joshi, S.C. Synthetic, magnetic, spectral, antimicrobial and antifertility studies of dioxomolybdenum(VI) unsymmetrical imine complexes having a N ∩ N donor system. Transit. Met. Chem. 2004, 29, 70–74. [Google Scholar] [CrossRef]
- Johnson, T.J.; Hedge, D.D. Esomeprazole: A clinical review. Am. J. Health-Syst. Pharm. 2002, 59, 1333–1339. [Google Scholar] [PubMed]
- Baluom, M.; Friedman, M.; Rubinstein, A. Improved intestinal absorption of sulpiride in rats with synchronized oral delivery systems. J. Control. Release 2001, 70, 139–147. [Google Scholar] [CrossRef]
- Loftsson, T. Drug solubilization by complexation. Int. J. Pharm. 2017, 531, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Ostrovskii, K.P.; Osipova, N.S.; Vanchugova, L.V.; Shipulo, E.V.; Pereverzeva, É.R.; Treshchalin, I.D.; Maksimenko, O.O.; Gel’perina, S.É. Use of proteins to increase the aqueous solubility of rifapentine. Pharm. Chem. J. 2016, 50, 407–412. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Agotegaray, M.; Gumilar, F.; Boeris, M.; Toso, R.; Minetti, A. Enhanced analgesic properties and reduced ulcerogenic effect of a mononuclear copper(II) complex with fenoprofen in comparison to the parent drug: Promising insights in the treatment of chronic inflammatory diseases. BioMed Res. Int. 2014, 2014, 505987. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.L.; Franz, K.J. Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.A.; Sadler, P.J. Photoactivatable metal complexes: From theory to applications in biotechnology and medicine. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371. [Google Scholar] [CrossRef] [PubMed]
- Price, J.D.; Piccariello, T.; Palmer, S. Metal-coordinated pharamceutical-reducing inter-subject variability with metal-coordinated pharmaceuticals: A case study with furosemide. Drug Dev. Deliv. 2014, 14, 63–68. [Google Scholar]
- Wang, H.; Hu, T.-L.; Wen, R.-M.; Wang, Q.; Bu, X.-H. In vitro controlled release of theophylline from metal–drug complexes. J. Mater. Chem. B 2013, 1, 3879–3882. [Google Scholar] [CrossRef]
- Martins, D.d.J.; Hanif Ur, R.; Rico, S.R.A.; Costa, I.d.M.; Santos, A.C.P.; Szszudlowski, R.G.; Silva, D.d.O. Interaction of chitosan beads with a copper–naproxen metallodrug. RSC Adv. 2015, 5, 90184–90192. [Google Scholar] [CrossRef]
- Mantion, A.; Massüger, L.; Rabu, P.; Palivan, C.; McCusker, L.B.; Taubert, A. Metal−peptide frameworks (MPFs): “Bioinspired” metal organic frameworks. J. Am. Chem. Soc. 2008, 130, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.H.; Wu, Y.S.; Liu, Y.C.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.-N.; Dang, Q.-Q.; Han, C.-Y.; Zhang, X.-M. An interpenetrated bioactive nonlinear optical MOF containing a coordinated quinolone-like drug and Zn(II) for pH-responsive release. Dalton Trans. 2015, 44, 1800–1804. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.J.; Runčevski, T.; Kapelewski, M.T.; Keitz, B.K.; Oktawiec, J.; Reed, D.A.; Mason, J.A.; Jiang, H.Z.H.; Colwell, K.A.; Legendre, C.M.; et al. Olsalazine-based metal–organic frameworks as biocompatible platforms for H2 adsorption and drug delivery. J. Am. Chem. Soc. 2016, 138, 10143–10150. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-J.; Xing, L.; Cui, P.-F.; Zhang, J.-L.; Zhu, Y.; Qiao, J.-B.; Lyu, J.-Y.; Zhang, M.; Luo, C.-Q.; Zhou, Y.-X.; et al. Transferrin-inspired vehicles based on pH-responsive coordination bond to combat multidrug-resistant breast cancer. Biomaterials 2017, 113, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Nong, J.; Nix, C.A.; Ji, H.-F.; Zhong, Y. Metal ion-assisted self-assembly of complexes for controlled and sustained release of minocycline for biomedical applications. Biofabrication 2015, 7, 015006. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.P.; Childs, S.L.; Giordano, J.; Iarriccio, A.; Cassidy, J.; Shet, M.S.; Mannion, R.; O’Donnell, E.; Park, A. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharm. Res. 2006, 23, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Song, F.; Wang, X.-H.; Cao, J.-Y.-Q.; Han, X.; Wang, X.-L.; Wang, Y.-Z. Ligand-metal-drug coordination based micelles for efficient intracellular doxorubicin delivery. RSC Adv. 2015, 5, 47629–47639. [Google Scholar] [CrossRef]
- Zayat, L.; Filevich, O.; Baraldo, L.M.; Etchenique, R. Ruthenium polypyridyl phototriggers: From beginnings to perspectives. Philos. Trans. Royal Soc. A 2013, 371, 20120330. [Google Scholar] [CrossRef] [PubMed]
- Zayat, L.; Calero, C.; Alborés, P.; Baraldo, L.; Etchenique, R. A new strategy for neurochemical photodelivery: Metal−ligand heterolytic cleavage. J. Am. Chem. Soc. 2003, 125, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Rial Verde, E.; Zayat, L.; Etchenique, R.; Yuste, R. Photorelease of GABA with visible light using an inorganic caging group. Front. Neural Circuits 2008, 2. [Google Scholar] [CrossRef] [PubMed]
- Lopes-dos-Santos, V.; Campi, J.; Filevich, O.; Ribeiro, S.; Etchenique, R. In vivo photorelease of GABA in the mouse cortex. Braz. J. Med. Biol Res. 2011, 44, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Knoll, J.D.; Albani, B.A.; Turro, C. Excited state investigation of a new Ru(II) complex for dual reactivity with low energy light. Chem. Commun. 2015, 51, 8777–8780. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Knoll, J.D.; Ancona, N.; Martin, P.D.; Turro, C.; Kodanko, J.J. Solid-phase synthesis as a platform for the discovery of new ruthenium complexes for efficient release of photocaged ligands with visible light. Inorg. Chem. 2015, 54, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreira, V.; Thorp-Greenwood, F.L.; Coogan, M.P. Application of d6 transition metal complexes in fluorescence cell imaging. Chem. Commun. 2009, 46, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Karaoun, N.; Renfrew, A.K. A luminescent ruthenium(II) complex for light-triggered drug release and live cell imaging. Chem. Commun. 2015, 51, 14038–14041. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Ghrayche, J.B.; Wei, J.; Renfrew, A.K. Photolabile ruthenium(II)–purine complexes: Phototoxicity, DNA binding, and light-triggered drug release. Eur. J. Inorg. Chem. 2017, 2017, 1679–1686. [Google Scholar] [CrossRef]
- Law, B.Y.K.; Qu, Y.Q.; Mok, S.W.F.; Liu, H.; Zeng, W.; Han, Y.; Gordillo-Martinez, F.; Chan, W.-K.; Wong, K.M.-C.; Wong, V.K.W. New perspectives of cobalt tris(bipyridine) system: Anti-cancer effect and its collateral sensitivity towards multidrug-resistant (MDR) cancers. Oncotarget 2017, 8, 55003–55021. [Google Scholar] [PubMed]
- Dabrowiak, J.C. Metals in Medicine, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; p. 221. [Google Scholar]
- Failes, T.W.; Cullinane, C.; Diakos, C.I.; Yamamoto, N.; Lyons, J.G.; Hambley, T.W. Studies of a cobalt(III) complex of the MMP inhibitor marimastat: A potential hypoxia-activated prodrug. Chem. Eur. J. 2007, 13, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.R.; Suntharalingam, K. Advances in cobalt complexes as anticancer agents. Dalton Trans. 2015, 44, 13796–13808. [Google Scholar] [CrossRef] [PubMed]
- Ware, D.C.; Palmer, H.R.; Pruijn, F.B.; Anderson, R.F.; Brothers, P.J.; Denny, W.A.; Wilson, W.R. Bis(dialkyl)dithiocarbamato cobalt(III) complexes of bidentate nitrogen mustards: Synthesis, reduction chemistry and biological evaluation as hypoxia-selective cytotoxins. Anti-Cancer Drug Des. 1998, 13, 81–103. [Google Scholar]
- Bustamante, F.L.S.; Metello, J.M.; de Castro, F.A.V.; Pinheiro, C.B.; Pereira, M.D.; Lanznaster, M. Lawsone dimerization in cobalt(III) complexes toward the design of new prototypes of bioreductive prodrugs. Inorg. Chem. 2013, 52, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Lago, A.B.; Pino-Cuevas, A.; Carballo, R.; Vazquez-Lopez, E.M. A new metal-organic polymeric system capable of stimuli-responsive controlled release of the drug ibuprofen. Dalton Trans. 2016, 45, 1614–1621. [Google Scholar] [CrossRef] [PubMed]







| Metal | Bioactive | Highlights | Ref. |
|---|---|---|---|
| Zn(II) | Pipemidic acid | pH-triggered release of bioactive from the bioactive interpenetrated MOF. | [26] |
| Mg(II) | Olsalazine, Phenethylamine | Enhanced biocompatible carrier with programmed co-delivery of an interpenetrated bioactive and entrapped model drug. | [27] |
| Fe(III) | Doxorubicin | Biomimetic delivery of an anti-cancer drug. | [28] |
| Mg(II) & Ca(II) | Minocycline HCl | Sustained release triggered by pathology-induced tissue acidosis. | [29] |
| Ru(II) | Purine model drug 4-aminopyridine GABA | Versatile light-activated carrier for neuroactives and econazole ligands with addition of imaging potential. New reports for purine anti-cancer model drugs. | [30] [39] [40] |
| Co(III) | Metalloproteinase | Controlled bioreductive release demonstrated. | [43] |
| Zn(II) | Ibuprofen | Release is based on ligand exchange mechanisms. | [47] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’bitsi-Ibouily, G.C.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. Outlook on the Application of Metal-Liganded Bioactives for Stimuli-Responsive Release. Molecules 2017, 22, 2065. https://doi.org/10.3390/molecules22122065
M’bitsi-Ibouily GC, Marimuthu T, Kumar P, Du Toit LC, Choonara YE, Kondiah PPD, Pillay V. Outlook on the Application of Metal-Liganded Bioactives for Stimuli-Responsive Release. Molecules. 2017; 22(12):2065. https://doi.org/10.3390/molecules22122065
Chicago/Turabian StyleM’bitsi-Ibouily, Gretta C., Thashree Marimuthu, Pradeep Kumar, Lisa C. Du Toit, Yahya E. Choonara, Pierre P. D. Kondiah, and Viness Pillay. 2017. "Outlook on the Application of Metal-Liganded Bioactives for Stimuli-Responsive Release" Molecules 22, no. 12: 2065. https://doi.org/10.3390/molecules22122065
APA StyleM’bitsi-Ibouily, G. C., Marimuthu, T., Kumar, P., Du Toit, L. C., Choonara, Y. E., Kondiah, P. P. D., & Pillay, V. (2017). Outlook on the Application of Metal-Liganded Bioactives for Stimuli-Responsive Release. Molecules, 22(12), 2065. https://doi.org/10.3390/molecules22122065