Nb-Based Zeolites: Efficient bi-Functional Catalysts for the One-Pot Synthesis of Succinic Acid from Glucose
Abstract
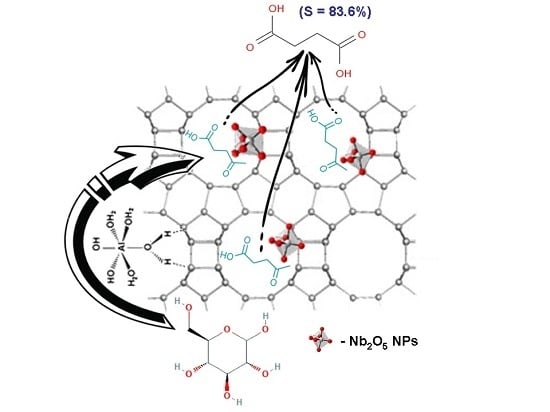
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterisation
2.2. Catalytic Behaviour
3. Materials and Methods
3.1. Catalysts Preparation
3.1.1. Conventional Activation Protocol: Na+/H+ Ion-Exchange
3.1.2. Synthesis of Nb-β Zeolites through the Dealumination of β Zeolites and Nb Insertion
3.2. Characterization Methods
3.3. Catalytic Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Christensen, C.H.; Rass-Hansen, J.; Marsden, C.C.; Taarning, E.; Egeblad, K. The renewable chemicals industry. ChemSusChem 2008, 1, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.P. Lignocellulosic conversion: An introduction to chemistry, process and economics. Biofuels Bioprod. Biorefin. 2007, 1, 39–48. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborra, S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011, 13, 520–540. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Shanks, B.H. Conversion of Biorenewable Feedstocks: New Challenges in Heterogeneous Catalysis. Ind. Eng. Chem. Res. 2010, 49, 10212–10217. [Google Scholar] [CrossRef]
- Bjerre, A.B.; Olesen, A.B.; Fernqvist, T.; Ploger, A.; Schmidt, A.S. Pretreatment of wheat straw using combined wet oxidation and alkaline hydrolysis resulting in convertible cellulose and hemicellulose. Biotechnol. Bioeng. 1996, 49, 568–577. [Google Scholar] [CrossRef]
- Klinke, H.B.; Ahring, B.K.; Schmidt, A.S.; Thomsen, A.B. Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour. Technol. 2002, 82, 15–26. [Google Scholar] [CrossRef]
- Schmidt, A.S.; Thomsen, A.B. Optimization of wet oxidation pretreatment of wheat straw. Bioresour. Technol. 1998, 64, 139–151. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Podolean, I.; Rizescu, C.; Bala, C.; Rotariu, L.; Parvulescu, V.I.; Coman, S.M.; Garcia, H. Unprecedented Catalytic Wet Oxidation of Glucose to Succinic Acid Induced by the Addition of n-Butylamine to a Ru(III) Catalyst. ChemSusChem 2016, 9, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Rizescu, C.; Podolean, I.; Cojocaru, B.; Parvulescu, V.I.; Coman, S.M.; Albero, J.; Garcia, H. RuCl3 Supported on N-Doped Graphene as a Reusable Catalyst for the One-Step Glucose Oxidation to Succinic Acid. ChemCatChem 2017, 9, 3314–3321. [Google Scholar] [CrossRef]
- Hunt, A.J. The importance of elemental sustainability and critical element recovery for the pharmaceutical industry. In Green and Sustainable Medicinal Chemistry: Methods, Tools and Strategies for the 21st Century Pharmaceutical Industry; Summerton, L., Sneddon, H.F., Jones, L.C., Clark, J.H., Eds.; The Royal Society of Chemistry: London, UK, 2016; Chapter 5; pp. 54–62. ISBN 978-1-78262-467-7. [Google Scholar]
- Rizescu, C.; Podolean, I.; Albero, J.; Parvulescu, V.I.; Coman, S.M.; Bucur, C.; Puche, M.; Garcia, H. N-Doped graphene as a metal-free catalyst for glucose oxidation to succinic acid. Green Chem. 2017, 19, 1999–2005. [Google Scholar] [CrossRef]
- Ding, D.; Wang, J.; Xi, J.; Liu, X.; Lu, G.; Wang, Y. Production of methyl levulinate from cellulose: Selectivity and mechanism study. Green Chem. 2014, 16, 3846–3853. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Marzo, M. Absence of expected side-reactions in the dehydration reaction of fructose to HMF in water over niobic acid catalyst. Catal. Commun. 2011, 12, 1122–1126. [Google Scholar] [CrossRef]
- García-Sancho, C.; Sádaba, I.; Moreno-Tost, R.; Mérida-Robles, J.; Santamaría-González, J.; López-Granados, M.; Maireles-Torres, P. Dehydration of Xylose to Furfural over MCM-41-Supported Niobium-Oxide Catalysts. ChemSusChem 2013, 6, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Coman, S.M.; Verziu, V.; Tirsoaga, A.; Jurca, B.; Teodorescu, C.; Kuncser, V.; Parvulescu, V.I.; Scholz, G.; Kemnitz, E. NbF5-AIF3 Catalysts: Design, Synthesis, and Application in Lactic Acid Synthesis from Cellulose. ACS Catal. 2015, 5, 3013–3026. [Google Scholar] [CrossRef]
- Podolean, I.; Anita, F.; Garcia, H.; Parvulescu, V.I.; Coman, S.M. Efficient magnetic recoverable acid-functionalized-carbon catalysts for starch valorization to multiple bio-chemicals. Catal. Today 2017, 279, 45–55. [Google Scholar] [CrossRef]
- Candu, N.; Anita, F.; Podolean, I.; Cojocaru, B.; Parvulescu, V.I.; Coman, S.M. Direct conversion of cellulose to α-hydroxy acids (AHAs) over Nb2O5-SiO2-coated magnetic nanoparticles. Green Process. Synth. 2017, 6, 255–264. [Google Scholar] [CrossRef]
- Ziolek, M. Niobium-containing catalysts—The state of the art. Catal. Today 2003, 78, 47–64. [Google Scholar] [CrossRef]
- Vermeiren, W.; Gilson, J.P. Impact of zeolites on the petroleum and petrochemical industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Meyers, R.A. Handbook of Petroleum Refining Processes, 3rd ed.; McGraw Hill: New York, NY, USA, 2004; 900p, ISBN 978-0071391092. [Google Scholar]
- Román-Leshkov, Y.; Davis, M.E. Activation of Carbonyl-Containing Molecules with Solid Lewis Acids in Aqueous Media. ACS Catal. 2011, 1, 1566–1580. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Román-Leshkov, Y. Domino Reaction Catalyzed by Zeolites with Brønsted and Lewis Acid Sites for the Production of γ-Valerolactone from Furfural. Angew. Chem. 2013, 125, 8180–8183. [Google Scholar] [CrossRef]
- D’Hondt, E.; Van de Vyver, S.; Sels, B.F.; Jacobs, P.A. Catalytic glycerol conversion into 1,2-propanediol in absence of added hydrogen. Chem. Commun. 2008, 6011–6012. [Google Scholar] [CrossRef] [PubMed]
- The Synthesis Commission. Available online: http://www.iza-online.org/synthesis/default.htm (accessed on 3 February 2017).
- Bhat, R.N.; Kumar, R. Synthesis of zeolite beta using silica gel as a source of SiO2. J. Chem. Technol. Biotechnol. 1990, 48, 453–466. [Google Scholar] [CrossRef]
- Perez-Pariente, J.; Martens, J.A.; Jacobs, P.A. Factors affecting the synthesis efficiency of zeolite BETA from aluminosilicate gels containing alkali and tetraethylammonium ions. Zeolites 1988, 8, 46–53. [Google Scholar] [CrossRef]
- Sazama, P.; Wichterlová, B.; Sklenák, Š.; Parvulescu, V.I.; Candu, N.; Sádovská, G.; Dědeček, J.; Klein, P.; Pashkova, V.; Šťastný, P. Acid and redox activity of template-free Al-rich H-BEA* and Fe-BEA* zeolites. J. Catal. 2014, 318, 22–33. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Peltre, M.J.; Massiani, P.; Davidson, A.; Che, M.; Sen, S.; Sivasankar, S. Incorporation of vanadium species in a dealuminated β zeolite. J. Chem. Soc. Chem. Commun. 1998, 87–88. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, D.; Zhuang, J.; Liu, X.; Liu, X.; Han, X.; Bao, X.; Chang, F.; Xu, L.; Liu, Z. On the acid-dealumination of USY zeolite: A solid state NMR investigation. J. Mol. Catal. A Chem. 2003, 194, 153–167. [Google Scholar] [CrossRef]
- Blasco, T.; Camblor, M.A.; Corma, A.; Esteve, P.; Guil, J.M.; Martinez, A.; Pedrigon-Melon, J.A.; Valencia, S. Direct Synthesis and Characterization of Hydrophobic Aluminum-Free Ti−Beta Zeolite. J. Phys. Chem. B 1998, 102, 75–88. [Google Scholar] [CrossRef]
- Tielens, F.; Shishido, T.; Dzwigaj, S. What Do the Niobium Framework Sites Look Like in Redox Zeolites? A Combined Theoretical and Experimental Investigation. J. Phys. Chem. C 2010, 114, 3140–3147. [Google Scholar] [CrossRef]
- Omegna, A.; Vasic, M.; van Bokhoven, J.A.; Pirngruber, G.; Prins, R. Dealumination and realumination of microcrystalline zeolite beta: An XRD, FTIR and quantitative multinuclear (MQ) MAS NMR study. Phys. Chem. Chem. Phys. 2004, 6, 447–452. [Google Scholar] [CrossRef]
- Corma, A.; Liabres i Xamena, F.X.; Prestipino, L.; Renz, C.M.; Valencia, S. Water Resistant, Catalytically Active Nb and Ta Isolated Lewis Acid Sites, Homogeneously Distributed by Direct Synthesis in a Beta Zeolite. J. Phys. Chem. C 2009, 113, 11306–11315. [Google Scholar] [CrossRef]
- Kawai, T.; Tsutsumi, K. A Study on the Surface Silanol Groups Developed by Hydrothermal and Acid Treatment of Faujasite Type Zeolites. J. Colloid Interface Sci. 1999, 212, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zukal, A.; Patzelová, V.; Lohse, U. Secondary porous structure of dealuminated Y zeolites. Zeolites 1986, 6, 133–136. [Google Scholar] [CrossRef]
- Beyerlein, R.A.; Choi-Feng, C.; Hall, J.B.; Huggins, B.J.; Ray, G.J. Effect of steaming on the defect structure and acid catalysis of protonated zeolites. Top. Catal. 1997, 4, 27–42. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Uozumi, R.; Satsuma, A. Enhanced production of hydroxymethylfurfural from fructose with solid acid catalysts by simple water removal methods. Catal. Commun. 2009, 10, 1849–1853. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Selective conversion of d-fructose to 5-hydroxymethylfurfural by ion-exchange resin in acetone/dimethyl sulfoxide solvent mixtures. Ind. Eng. Chem. Res. 2008, 47, 9234–9239. [Google Scholar] [CrossRef]
- Herbst, A.; Janiak, C. Selective glucose conversion to 5-hydroxymethylfurfural (5-HMF) instead of levulinic acid with MIL-101Cr MOF-derivatives. New J. Chem. 2016, 40, 7958–7967. [Google Scholar] [CrossRef]
- Saroha, A.K.; Vashishtha, M.; Roy, S. Catalytic wet air oxidation of oxalic acid using platinum catalysts in bubble column reactor: A review. J. Eng. Sci. Technol. Rev. 2010, 3, 95–107. [Google Scholar]
- Ziolek, M.; Sobczak, I. The role of niobium component in heterogeneous catalysts. Catal. Today 2017, 285, 211–225. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I.; Decyk, P.; Sobanska, K.; Pietrzyk, P.; Sojka, Z. Search for reactive intermediates in catalytic oxidation with hydrogen peroxide over amorphous niobium(V) and tantalum(V) oxides. Appl. Catal. B Environ. 2015, 164, 288–296. [Google Scholar] [CrossRef]
- Ennaert, T.; Geboers, J.; Gobechiya, E.; Courtin, C.M.; Kurttepeli, M.; Houthoofd, K.; Kirschhock, C.E.; Magusin, A.P.C.M.; Bals, M.S.; Jacobs, P.A.; et al. Conceptual Frame Rationalizing the Self-Stabilization of H-USY Zeolites in Hot Liquid Water. ACS Catal. 2015, 5, 754–768. [Google Scholar] [CrossRef]
- Ortiz-Iniesta, M.J.; Heeres, H.J.; Cabrera, I.M. Direct activation of microcrystalline zeolites. Microporous Mesoporous Mater. 2013, 171, 208–214. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| H-Beta Sample | Si/Al Molar Ratio | Crystalline Phase | Crystallinity (%) |
|---|---|---|---|
| H-β6 | 6 | Beta zeolite | 83.1 |
| H-β12 | 12 | Beta zeolite | 99.5 |
| H-(β18 and β37) | 18 and 37 | Beta zeolite | 100 |
| Entry | Sample | θ, ° | d302, Å |
|---|---|---|---|
| 1 | DeAl-β12 (nitric acid) | 22.68 | 3.917 |
| 2 | Nb(0.02)-β12 | 22.57 | 3.936 |
| 3 | Nb(0.05)-β12 | 22.66 | 3.921 |
| 4 | DeAl-β18 (nitric acid) | 22.67 | 3.917 |
| 5 | Nb(0.02)-β18 | 22.61 | 3.929 |
| 6 | Nb(0.05)-β18 | 22.63 | 3.926 |
| 7 | DeAl-β18 (oxalic acid) | 22.75 | 3.906 |
| 8 | Nb(0.05)-β18O | 22.57 | 3.936 |
| 9 | DeAl-β37 (oxalic acid) | 22.71 | 3.912 |
| 10 | Nb(0.05)-β37O | 22.38 | 3.969 |
| Entry | Catalyst | X, % | S, % | |||
|---|---|---|---|---|---|---|
| LA | GA | LevA | SA | |||
| 1 | Nb(0.02)-β12 | 86.2 | 3.1 | 5.9 | 19.0 | 52.0 |
| 2 | Nb(0.05)-β12 | 100 | 1.0 | 3.1 | 5.3 | 62.3 |
| 3 | Nb(0.05)-β18 | 100 | 3.1 | 2.1 | 16.6 | 72.3 |
| 4 | Nb(0.05)-β37.5 | 100 | 0.6 | 0.9 | 3.5 | 83.6 |
| 5 * | Nb(0.05)-β37.5 | 98.7 | 2.8 | 4.3 | 22.7 | 58.3 |
| 6 | Nb(0.05)-β37.5O | 100 | 0 | 1.3 | 10.2 | 70.1 |
| Entry | Catalyst | X, % | SSA, % |
|---|---|---|---|
| 1 | Fresh catalyst (1st cycle) | 86.4 | 26.1 |
| 2 * | After 2 h from the catalyst separation | 86.5 | 26.2 |
| 3 | 2nd cycle | 86.0 | 26.0 |
| 4 | 3rd cycle | 86.2 | 26.1 |
| 5 | 4th cycle | 85.8 | 25.4 |
| The Type of the Zeolite Samples | Cation | Si/Al Ratio | The Notation (Symbols) |
|---|---|---|---|
| Beta | Na+ | 6 | Na+-β6 |
| Beta | NH4+ | 12 | NH4+-β12 |
| Beta | NH4+ | 18 | NH4+-β18 |
| Beta | NH4+ | 37.5 | NH4+-β37 |
| Zeolite Type | Si/Al Ratio | Dealumination Treatment Acid | Nb Content, mol % | Denoted Nb-Based Materials |
|---|---|---|---|---|
| Beta | 12 | Nitric | 0.02 | Nb(0.02)-β12 |
| Beta | 12 | Nitric | 0.05 | Nb(0.05)-β12 |
| Beta | 18 | Nitric | 0.02 | Nb(0.02)-β18 |
| Beta | 18 | Nitric | 0.05 | Nb(0.05)-β18 |
| Beta | 18 | Oxalic | 0.05 | Nb(0.05)-β18O |
| Beta | 37.5 | Oxalic | 0.05 | Nb(0.05)-β37O |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Fergani, M.; Candu, N.; Coman, S.M.; Parvulescu, V.I. Nb-Based Zeolites: Efficient bi-Functional Catalysts for the One-Pot Synthesis of Succinic Acid from Glucose. Molecules 2017, 22, 2218. https://doi.org/10.3390/molecules22122218
El Fergani M, Candu N, Coman SM, Parvulescu VI. Nb-Based Zeolites: Efficient bi-Functional Catalysts for the One-Pot Synthesis of Succinic Acid from Glucose. Molecules. 2017; 22(12):2218. https://doi.org/10.3390/molecules22122218
Chicago/Turabian StyleEl Fergani, Magdi, Natalia Candu, Simona M. Coman, and Vasile I. Parvulescu. 2017. "Nb-Based Zeolites: Efficient bi-Functional Catalysts for the One-Pot Synthesis of Succinic Acid from Glucose" Molecules 22, no. 12: 2218. https://doi.org/10.3390/molecules22122218
APA StyleEl Fergani, M., Candu, N., Coman, S. M., & Parvulescu, V. I. (2017). Nb-Based Zeolites: Efficient bi-Functional Catalysts for the One-Pot Synthesis of Succinic Acid from Glucose. Molecules, 22(12), 2218. https://doi.org/10.3390/molecules22122218