Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal
Abstract
:1. Introduction
2. Results and Discussion
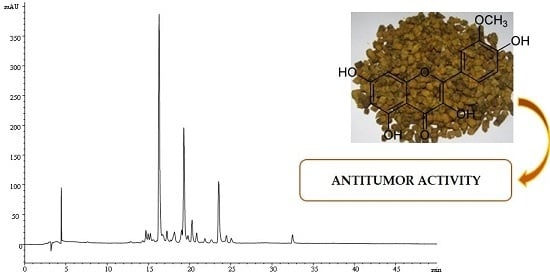
2.1. Chromatographic Profile of the BB
2.2. Antitumoral Activity of the BB Samples
3. Materials and Methods
3.1. Samples Collection
3.2. Standards and Reagents
3.3. Extracts Preparation
3.4. Characterization of the Extracts by HPLC-DAD-ESI/MS
3.5. Evaluation of In Vitro Cytotoxic Properties of the Extracts
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gilliam, M. Microbiology of pollen and bee: The yeasts. Apidologie 1979, 10, 43–53. [Google Scholar] [CrossRef]
- Markiewicz-Żukowska, R.; Naliwajko, S.K.; Bartosiuk, E.; Moskwa, J.; Isidorov, V.; Soroczyńska, J.; Borawska, M.H. Chemical composition and antioxidant activity of beebread, and its influence on the glioblastoma cell line (U87MG). J. Apic. Sci. 2013, 57, 147–157. [Google Scholar] [CrossRef]
- Nagai, T.; Nagashima, T.; Myoda, T.; Inoue, R. Preparation and functional properties of extracts from bee bread. Food/Nahrung 2004, 48, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Durán, X.A.; Mardones, I.Q.; Gutiérrez, M.M.; Ulloa, D.M. Polifenoles totales en pan de abeja (Apis mellifera L.) de colmenas de la Región de La Araucanía. Idesia (Arica) 2014, 32, 107–111. [Google Scholar] [CrossRef]
- Abouda, Z.; Zerdani, I.; Kalalou, I.; Faid, M.; Ahami, M.T. The antibacterial activity of Moroccan bee bread and bee-pollen (fresh and dried) against pathogenic bacteria. Res. J. Microbiol. 2011, 6, 376–384. [Google Scholar]
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Antibacterial activity of honey and beebread of different origin against S. aureus and S. epidermidis. Food Technol. Biotechnol. 2007, 45, 201–208. [Google Scholar]
- Borawska, M.H.; Markiewicz-Żukowska, R.; Naliwajko, S.K.; Moskwa, J.; Bartosiuk, E.; Socha, K.; Surażyńsk, A.; Kochanowicz, J.; Mariak, Z. The Interaction of Bee Products With Temozolomide in Human Diffuse Astrocytoma, Glioblastoma Multiforme and Astroglia Cell Lines. Nutr. Cancer 2014, 66, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Giroud, B.; Vauchez, A.; Vulliet, E.; Wiest, L.; Buleté, A. Trace level determination of pyrethroid and neonicotinoid insecticides in beebread using acetonitrile-based extraction followed by analysis with ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1316, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Isidorova, A.G.; Sczczepaniak, L.; Czyżewska, U. Gas chromatographic-mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. Food Chem. 2009, 115, 1056–1063. [Google Scholar] [CrossRef]
- Tavdidishvili, D.; Khutsidze, T.; Pkhakadze, M.; Vanidze, M.; Kalandia, A. Flavonoids in Georgian bee bread and bee pollen. J. Chem. 2014, 8, 676–681. [Google Scholar]
- Markham, K.R.; Mitchell, K.A.; Campos, M. An unusually lipophilic flavonol glycoside from Ranunculus sardous pollen. Phytochemistry 1997, 45, 203–204. [Google Scholar] [CrossRef]
- Markham, K.R.; Campos, M. 7- and 8-O-Methylherbacetin-3-O-sophorosides from bee pollens and some structure/activity observations. Phytochemistry 1996, 43, 763–767. [Google Scholar] [CrossRef]
- Campos, M.; Markham, K.R.; Mitchell, K.A.; Cunha, A.P. An approach to the characterization of bee pollens via their flavonoid/phenolic profiles. Phytochem. Anal. 1997, 8, 181–185. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Anti Cancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Food 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Abreu, R.M.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.J.R. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno [3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sample Availability: BB samples are available from the authors.



| Peak | Rt (min) | λmax (nm) | Molecular ion [M − H]− (m/z) | MS2 (m/z) | Identification |
|---|---|---|---|---|---|
| 1 | 14.3 | 358 | 625 | 317 (100) | Myricetin-3-O-rutinoside |
| 2 | 14.7 | 350 | 771 | 609 (100), 463 (9), 301 (23) | Quercetin-O-hexosyl-O-rutinoside |
| 3 | 14.9 | 346 | 755 | 593 (100), 447 (21), 285 (34) | Kaempferol-O-hexosyl-O-rutinoside |
| 4 | 15.3 | 350 | 625 | 463 (100), 301 (48) | Quercetin-O-hexosyl-O-hexoside |
| 5 | 16.3 | 350 | 785 | 623 (100), 477 (16), 315 (30) | Isorhamnetin-O-hexosyl-O-rutinoside |
| 6 | 16.3 | 350 | 639 | 315 (18), 300 (21) | Methyl herbacetrin-O-dihexoside |
| 7 | 16.5 | 354 | 479 | 317 (100) | Myricetin-3-O-glucoside |
| 8 | 16.7 | 358 | 595 | 301 (100) | Quercetin-O-pentosyl-hexoside |
| 9 | 16.8 | 356 | 771 | 301 (100) | Quercetin-O-hexosyl-rutinoside |
| 10 | 17.3 | 358 | 609 | 301 (100) | Quercetin 3-O-rutinoside |
| 11 | 17.4 | 350 | 785 | 315 (32), 300 (20) | Methyl herbacetrin-O-hexosyl-rutinoside |
| 12 | 17.7 | 348 | 609 | 285 (100) | Kaempferol-O-dihexoside |
| 13 | 18.2 | 352 | 623 | 315 (36), 300 (22) | Methyl herbacetrin-3-O-rutinoside |
| 14 | 18.4 | 350 | 639 | 315 (29), 300 (14) | Methyl herbacetrin-O-dihexoside |
| 15 | 19.0 | 350 | 755 | 285 (100) | Kaempferol-O-hexosyl-rutinoside |
| 16 | 19.3 | 356 | 609 | 315 (100) | Isorhamnetin-O-pentosyl-hexoside |
| 17 | 19.6 | 354 | 785 | 315 (100) | Isorhamnetin-O-hexosyl-rutinoside |
| 18 | 19.8 | 348 | 593 | 285 (100) | Kaempferol-3-O-rutinoside |
| 19 | 20.3 | 356 | 463 | 301 (100) | Quercetin-3-O-glucoside |
| 20 | 20.9 | 354 | 609 | 315 (100) | Isorhamnetin-O-pentosyl-hexoside |
| 21 | 21.9 | 356 | 609 | 315 (100) | Isorhamnetin-O-pentosyl-hexoside |
| 22 | 22.6 | 350 | 635 | 285 (100) | Acetyl kaempferol-O-deoxyhexosyl-hexoside |
| 23 | 22.7 | 346 | 477 | 315 (50), 300 (33) | Methyl herbacetrin-3-O-glucoside |
| 24 | 23.2 | 356 | 477 | 331 (19), 315 (32) | Laricitrin-3-O-rhamnoside |
| 25 | 23.5 | 356 | 623 | 315 (100) | Isorhamnetin-3-O-rutinoside |
| 26 | 24.2 | 350 | 447 | 301 (100) | Quercetin-3-O-rhamnoside |
| 27 | 24.5 | 348 | 563 | 285 (100) | Kaempferol-O-pentosyl-deoxyhexoside |
| 28 | 25.1 | 356 | 477 | 315 (100) | Isorhamnetin-3-O-glucoside |
| 29 | 26.7 | 350 | 489 | 285 (100) | Acetyl kaempferol-O-hexoside |
| 30 | 28.8 | 346 | 431 | 285 (100) | Kaempferol-3-O-rhamnoside |
| 31 | 29.9 | 350 | 461 | 315 (100) | Isorhamnetin-3-O-rhamnoside |
| 32 | 32.5 | 356 | 519 | 315 (100) | Acetyl isorhamnetin-O-hexoside |
| BB1 | BB2 | BB3 | BB4 | BB5 | BBC | Normal Distribution 1 | Homoscedasticity 2 | Differences among Means 3 | |
|---|---|---|---|---|---|---|---|---|---|
| Myricetin-3-O-rutinoside | 41 ± 3c | nd | 322 ± 7a | nd | 38 ± 4c | 118 ± 4b | 0.002 | 0.719 | <0.001 |
| Quercetin-O-hexosyl-O-rutinoside | 156 ± 8 | nd | nd | nd | nd | nd | - | - | - |
| Kaempferol- O-hexosyl-O-rutinoside | 69 ± 1 | nd | nd | nd | nd | nd | - | - | - |
| Quercetin-O-hexosyl-O-hexoside | 129 ± 5c | 211 ± 6b | nd | 127 ± 4c | 74 ± 3d | 1580 ± 31a | <0.001 | 0.089 | <0.001 |
| Isorhamnetin-O-hexosyl-O-rutinoside | 2615 ± 54 | nd | nd | nd | nd | nd | - | - | - |
| Methyl herbacetrin-O-dihexoside | nd | 622 ± 25a | 70 ± 3d | 460 ± 3b | 192 ± 1c | nd | 0.046 | 0.084 | <0.001 |
| Myricetin-3-O-glucoside | nd | nd | 36 ± 2 | nd | nd | nd | - | - | - |
| Quercetin-O-pentosyl-hexoside | 100 ± 5 | nd | nd | nd | nd | 139 ± 1 | 0.023 | 0.148 | <0.001 |
| Quercetin-O-hexosyl-rutinoside | nd | 314 ± 6b | 106 ± 9c | 367 ± 1a | nd | nd | 0.006 | 0.290 | <0.001 |
| Quercetin 3-O-rutinoside | 158 ± 3c | 88 ± 5e | 312 ± 5b | 105 ± 6d | 91 ± 2e | 377 ± 7a | 0.001 | 0.688 | <0.001 |
| Methyl herbacetrin-O-hexosyl-rutinoside | nd | nd | nd | nd | 217 ± 1 | nd | - | - | - |
| Kaempferol-O-dihexoside | nd | 91 ± 6c | 246 ± 8b | 83 ± 4c | 108 ± 1c | 1167 ± 30a | <0.001 | 0.094 | <0.001 |
| Methyl herbacetrin-3-O-rutinoside | 186 ± 25c | nd | 71 ± 1d | 435 ± 5a | 225 ± 7b | nd | 0.037 | 0.119 | <0.001 |
| Methyl herbacetrin-O-dihexoside | nd | nd | 39 ± 3d | 164 ± 13b | 268 ± 11a | 105 ± 5c | 0.152 | 0.424 | <0.001 |
| Kaempferol-O-hexosyl-rutinoside | 212 ± 10d | 3597 ± 69b | 403 ± 1c | 3755 ± 46a | nd | 130 ± 2e | <0.001 | 0.095 | <0.001 |
| Isorhamnetin-O-pentosyl-hexoside | 1448 ± 37 | nd | nd | nd | nd | nd | - | - | - |
| Isorhamnetin-O-hexosyl-rutinoside | nd | 103 ± 1 | nd | 43 ± 3 | nd | nd | 0.009 | 0.208 | <0.001 |
| Kaempferol-3-O-rutinoside | 62 ± 7de | 94 ± 21d | 355 ± 10c | 56 ± 4e | 815 ± 16b | 1627 ± 32a | <0.001 | 0.300 | <0.001 |
| Quercetin-3-O-glucoside | 248 ± 5a | 52 ± 8e | 236 ± 1b | 53 ± 3e | 177 ± 3c | 72 ± 1d | 0.001 | 0.249 | <0.001 |
| Isorhamnetin-O-pentosyl-hexoside | 94 ± 8 | nd | nd | nd | nd | nd | - | - | - |
| Isorhamnetin-O-pentosyl-hexoside | 30 ± 2 | nd | 47 ± 3 | nd | nd | nd | 0.081 | 0.743 | <0.001 |
| Acetyl kaempferol-O-deoxyhexosyl-hexoside | nd | nd | 20 ± 1 | nd | 11 ± 1 | nd | 0.037 | 0.639 | <0.001 |
| Methyl herbacetrin-3-O-glucoside | tr | 32 ± 4d | 53 ± 6c | 224 ± 10a | 138 ± 17b | nd | 0.033 | 0.383 | <0.001 |
| Laricitrin-3-O-rhamnoside | nd | nd | 125 ± 5 | nd | nd | nd | - | - | - |
| Isorhamnetin-3-O-rutinoside | 836 ± 35 | nd | nd | nd | nd | nd | - | - | - |
| Quercetin-3-O-rhamnoside | tr | 280 ± 22c | 3029 ± 72a | 168 ± 19d | 2001 ± 17b | 190 ± 7d | 0.001 | 0.236 | <0.001 |
| Kaempferol-O-pentosyl-deoxyhexoside | 82 ± 6 | nd | nd | nd | nd | nd | - | - | - |
| Isorhamnetin-3-O-glucoside | 140 ± 1b | nd | 199 ± 1a | nd | 118 ± 4c | 64 ± 2d | 0.103 | 0.269 | <0.001 |
| Acetyl kaempferol-O-hexoside | nd | nd | nd | nd | nd | 22 ± 3 | - | - | - |
| Kaempferol-3-O-rhamnoside | nd | nd | 141 ± 12 | nd | 29 ± 10 | nd | 0.038 | 0.747 | <0.001 |
| Isorhamnetin-3-O-rhamnoside | nd | 73 ± 10c | 670 ± 44a | nd | 232 ± 14b | nd | 0.022 | 0.234 | <0.001 |
| Acetyl isorhamnetin-O-hexoside | 197 ± 12 | nd | nd | nd | nd | nd | - | - | - |
| Total flavonoids | 6802 ± 204a | 5557 ± 179d | 6480 ± 128b | 6040 ± 76c | 4733 ± 106e | 5593 ± 118d | 0.417 | 0.804 | <0.001 |
| Human Tumor Cell Lines | Non-Tumor Porcine Liver Cells | ||||
|---|---|---|---|---|---|
| MCF-7 | NCI-H460 | HeLa | HepG2 | PLP2 | |
| BB1 | 186 ± 6a | >400 | 345 ± 13a | >400 | >400 |
| BB2 | 84 ± 3c | >400 | >400 | >400 | >400 |
| BB3 | 164 ± 4b | 253 ± 10a | 225 ± 12bc | 67 ± 1 | >400 |
| BB4 | >400 | 85 ± 5b | 209 ± 21c | >400 | >400 |
| BB5 | >400 | 68 ± 8b | 276 ± 18b | >400 | >400 |
| BBC | >400 | >400 | 366 ± 7a | >400 | >400 |
| Ellipticine | 0.45 ± 0.02 | 0.74 ± 0.01 | 0.55 ± 0.03 | 1.61 ± 0.07 | 1.06 ± 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobral, F.; Calhelha, R.C.; Barros, L.; Dueñas, M.; Tomás, A.; Santos-Buelga, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal. Molecules 2017, 22, 248. https://doi.org/10.3390/molecules22020248
Sobral F, Calhelha RC, Barros L, Dueñas M, Tomás A, Santos-Buelga C, Vilas-Boas M, Ferreira ICFR. Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal. Molecules. 2017; 22(2):248. https://doi.org/10.3390/molecules22020248
Chicago/Turabian StyleSobral, Filipa, Ricardo C. Calhelha, Lillian Barros, Montserrat Dueñas, Andreia Tomás, Celestino Santos-Buelga, Miguel Vilas-Boas, and Isabel C. F. R. Ferreira. 2017. "Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal" Molecules 22, no. 2: 248. https://doi.org/10.3390/molecules22020248
APA StyleSobral, F., Calhelha, R. C., Barros, L., Dueñas, M., Tomás, A., Santos-Buelga, C., Vilas-Boas, M., & Ferreira, I. C. F. R. (2017). Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal. Molecules, 22(2), 248. https://doi.org/10.3390/molecules22020248