Glycerol as Precursor of Organoselanyl and Organotellanyl Alkynes
Abstract
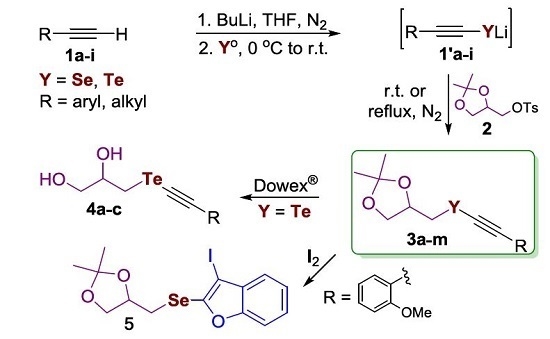
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. General Procedure for Synthesis of Organoselanyl Alkynes 3a–h
Analytical Data of Products 3a–h
3.3. General Procedure for the Synthesis of Organotellanyl Alkynes 3i–m
Analytical Data of Products 3i–m
3.4. General Procedure for the Synthesis of 3-(Organotellanyl)propane-1,2-diol 4a–c
Analytical Data of Products 4a–c
3.5. Procedure for the Synthesis of the 3-Iodo-2-(2,2-dimethyl-1,3-dioxolanylmethyl)selenanylbenzo[b]furan 5
Analytical Data of Product 5
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wirth, T. Organoselenium Chemistry—Modern Developments in Organic Synthesis; Topics in Current Chemistry; Springer: Heidelberg, Germany, 2000; Volume 208. [Google Scholar]
- Zeni, G.; Lüdtke, D.S.; Panatieri, R.B.; Braga, A.L. Vinylic Tellurides: From Preparation to Their Applicability in Organic Synthesis. Chem. Rev. 2006, 106, 1032–1076. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of Vinyl Selenides. Chem. Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.H.; Zeni, G. Vinyl Selenides in Patai’s Chemistry of Functional Groups; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Palomba, M.; Bagnoli, L.; Marini, F.; Santi, C.; Sancineto, L. Recent advances in the chemistry of vinylchalcogenides. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 235–244. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Hazra, A.; Chakrabarty, R.; Fun, H.-K. Recognition of carboxylate anions and carboxylic acids by selenium-based new chromogenic fluorescent sensor: A remarkable fluorescence enhancement of hindered carboxylates. Org. Lett. 2009, 11, 4350–4353. [Google Scholar] [CrossRef] [PubMed]
- Rampon, D.S.; Rodembush, F.S.; Schneider, J.M.F.M.; Bechtold, I.H.; Gonçalves, P.F.B.; Merlo, A.A.; Schneider, P.H. Novel selenoesters fluorescent liquid crystalline exhibiting a rich phase polymorphism. J. Mater. Chem. 2010, 20, 715–722. [Google Scholar] [CrossRef]
- Samb, I.; Bell, J.; Toullec, P.Y.; Michelet, V.; Leray, I. Fluorescent phosphane selenide as efficient mercury chemodosimeter. Org. Lett. 2011, 13, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.-W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Santoro, S.; Azeredo, J.B.; Nascimento, V.; Sancineto, L.; Braga, A.L.; Santi, C. The green side of the moon: Ecofriendly aspects of organoselenium chemistry. RSC Adv. 2014, 4, 31521–31535. [Google Scholar] [CrossRef]
- Santi, C. Organoselenium Chemistry between Synthesis and Biochemistry, 1st ed.; Bentham eBooks: Perugia, Italy, 2014. [Google Scholar]
- Barcellos, A.M.; Abenante, L.; Sarro, M.T.; Leo, I.D.; Lenardão, E.J.; Perin, G.; Santi, C. New prospective for redox modulation mediated by organoselenium and organotellurium compounds. Curr. Org. Chem. 2017, 21, 1–18. [Google Scholar] [CrossRef]
- Pérez-Balado, C.; Markó, I.E. 1-Iodo-1-selenoalkenes as versatile alkene 1,1-dianion equivalents. Novel connective approach towards the tetrahydropyran subunit of polycavernoside A. Tetrahedron 2006, 62, 2331–2349. [Google Scholar] [CrossRef]
- Liu, C.-R.; Yang, F.-L.; Jin, Y.-Z.; Ma, X.-T.; Cheng, D.-J.; Li, N.; Tian, S.-K. Catalytic regioselective synthesis of structurally diverse indene derivatives from n-benzylic sulfonamides and disubstituted alkynes. Org. Lett. 2010, 12, 3832–3835. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, T.; Ogawa, A. Palladium-catalyzed alkynylselenation of acetylenedicarboxylates leading to enyne selenides and application to synthesis of multisubstituted aryl selenides. Tetrahedron Lett. 2010, 51, 3538–3541. [Google Scholar] [CrossRef]
- Okoronkwo, A.E.; Godoi, B.; Schumacher, R.F.; Neto, J.S.S.; Luchese, C.; Prigol, M.; Nogueira, C.W.; Zeni, G. Csp3-tellurium copper cross-coupling: Synthesis of alkynyl tellurides a novel class of antidepressive-like compounds. Tetrahedron Lett. 2009, 50, 909–915. [Google Scholar] [CrossRef]
- Savegnago, L.; Borges, V.C.; Alves, D.; Jesse, C.R.; Rocha, J.B.T.; Nogueira, C.W. Evaluation of antioxidant activity and potential toxicity of 1-buthyltelurenyl-2-methylthioheptene. Life Sci. 2006, 79, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Ávila, D.S.; Gubert, P.; Palma, A.; Colle, D.; Alves, D.; Nogueira, C.W.; Rocha, J.B.T.; Soares, F.A.A. An organotellurium compound with antioxidant activity against excitotoxic agents without neurotoxic effects in brain of rats. Brain Res. Bull. 2008, 76, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Manarin, F.; Roehrs, J.A.; Gay, R.M.; Brandão, R.; Menezes, P.H.; Nogueira, C.W.; Zeni, G. Electrophilic cyclization of 2-chalcogenealkynylanisoles: Versatile access to 2-chalcogen-benzo[b]furans. J. Org. Chem. 2009, 74, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.C.; Back, D.F.; Zeni, G. Chalcogenoalkynes: Precursors for the regioselective preparation of 2-chalcogeno-1-halonaphthalenes through [4 + 2] cycloaddition. Eur. J. Org. Chem. 2012, 4574–4579. [Google Scholar] [CrossRef]
- Lara, R.G.; Borges, E.L.; Lenardão, E.J.; Alves, D.; Jacob, R.G.; Perin, G. Addition of thiols to phenylselenoalkynes using KF/alumina under solvent-free conditions. J. Braz. Chem. Soc. 2010, 21, 2125–2129. [Google Scholar] [CrossRef]
- Perin, G.; Borges, E.L.; Alves, D. Highly stereoselective method to prepare bis-phenylchalcogen alkenes via addition of chalcogenolate to phenylseleno alkynes. Tetrahedron Lett. 2012, 53, 2066–2069. [Google Scholar] [CrossRef]
- Rampon, D.S.; Giovenardi, R.; Silva, T.L.; Rambo, R.S.; Merlo, A.A.; Schneider, P.H. Chalcogenoacetylenes obtained by indium(III) catalysis: Dual catalytic activation of diorgano dichalcogenides and Csp-H bonds. Eur. J. Org. Chem. 2011, 7066–7070. [Google Scholar] [CrossRef]
- Godoi, M.; Ricardo, E.W.; Frizon, T.E.; Rocha, M.S.T.; Singh, D.; Paixão, M.W.; Braga, A.L. An efficient synthesis of alkynyl selenides and tellurides from terminal acetylenes and diorganyl diselenides or ditellurides catalyzed by recyclable copper oxide nanopowder. Tetrahedron 2012, 68, 10426–10430. [Google Scholar] [CrossRef]
- Ahammed, S.; Bhadra, S.; Kundu, D.; Sreedhar, B.; Ranu, B.C. An efficient and general procedure for the synthesis of alkynyl chalcogenides (selenides and tellurides) by alumina-supported Cu(II)-catalyzed reaction of alkynyl bromides and diphenyl dichalcogenides. Tetrahedron 2012, 68, 10542–10549. [Google Scholar] [CrossRef]
- Movasssagh, B.; Yousefi, A.; Momeni, B.Z.; Heydari, S. A general and highly efficient protocol for the synthesis of chalcogenoacetylenes by copper(I)-terpyridine catalyst. Synlett 2014, 25, 1385–1390. [Google Scholar] [CrossRef]
- Godoi, M.; Liz, D.G.; Ricardo, E.W.; Rocha, M.S.T.; Azeredo, J.B.; Braga, A.L. Magnetite (Fe3O4) nanoparticles: An efficient and recoverable catalyst for the synthesis of alkynyl chalcogenides (selenides and tellurides) from terminal acetylenes and diorganyl dichalcogenides. Tetrahedron 2014, 70, 3349–3354. [Google Scholar] [CrossRef]
- Mohammadi, E. Movassagh, B. Cryptand-22 as an efficient ligand for the copper-catalyzed cross-coupling reaction of diorgano dichalcogenides with terminal alkynes leading to the synthesis of alkynyl chalcogenides. Tetrahedron Lett. 2014, 55, 1613–1615. [Google Scholar] [CrossRef]
- Alves, D.; Sachini, M.; Jacob, R.G.; Lenardão, E.J.; Contreira, M.E.; Savegnago, L.; Perin, G. Synthesis of (Z)-organylthioenynes using KF/Al2O3/solvent as recyclable system. Tetrahedron Lett. 2011, 52, 133–135. [Google Scholar] [CrossRef]
- Len, C.; Luque, R. Continuous flow transformations of glycerol to valuable products: An overview. Sustain. Chem. Process. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Mori, K. Pheromone synthesis. Part 253: Synthesis of the racemates and enantiomers of triglycerides of male Drosophila fruit flies with special emphasis on the preparation of enantiomerically pure 1-monoglycerides. Tetrahedron 2012, 68, 8441–8449. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Pagano, D.; Fontana, A. An efficient and versatile chemical synthesis of bioactive glyco-glycerolipids. Tetrahedron Lett. 2012, 53, 879–881. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Malladi, S.S.; Warshakoon, H.J.; Kimbrell, M.R.; Amolins, M.W.; Ukani, R.; Datta, A.; David, S.A. Structure–activity relationships in toll-like receptor-2 agonistic diacylthioglycerol lipopeptides. J. Med. Chem. 2010, 53, 3198–3213. [Google Scholar] [CrossRef] [PubMed]
- Goubert, M.; Canet, I.; Sinibaldi, M.-E. An expedient route to new spiroheterocycles: Synthesis and structural studies. Eur. J. Org. Chem. 2006, 4805–4812. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.; Yamada, K.; Asami, T.; Yoshizawa, Y. Synthesis of novel brassinosteroid biosynthesis inhibitors based on the ketoconazole scaffold. Bioorg. Med. Chem. Lett. 2012, 22, 1625–1628. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Hirschfeld, D.; Tan, Y.-C.; Hogg, J.H.; Peltz, G.; Avnur, Z.; Dunten, P. Discovery of potent and bioavailable GSK-3β inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.; Borges, E.L.; Duarte, J.E.G.; Webber, R.; Jacob, R.G.; Lenardão, E.J. Glycerol as renewable resource in the synthesis of thioethers using KF/Al2O3. Curr. Green. Chem. 2014, 1, 115–120. [Google Scholar] [CrossRef]
- Nobre, P.C.; Borges, E.L.; Silva, C.M.; Casaril, A.M.; Martinez, D.M.; Lenardão, E.J.; Alves, D.; Savegnago, L.; Perin, G. Organochalcogen compounds from glycerol: Synthesis of new antioxidants. Bioorg. Med. Chem. 2014, 22, 6242–6249. [Google Scholar] [CrossRef] [PubMed]
- Borges, E.L.; Peglow, T.J.; Silva, M.S.; Jacoby, C.G.; Schneider, P.H.; Lenardão, E.J.; Jacob, R.G.; Perin, G. Synthesis of enantiomerically pure bis(2,2-dimethyl-1,3-dioxolanylmethyl)chalcogenides and dichalcogenides. New J. Chem. 2016, 40, 2321–2326. [Google Scholar] [CrossRef]
- Soares, L.K.; Silva, R.B.; Peglow, T.J.; Silva, M.S.; Jacob, R.G.; Alves, D.; Perin, G. Selective synthesis of vinyl- or alkynyl chalcogenides from glycerol and their water-soluble derivatives. ChemistrySelect 2016, 1, 2009–2013. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2–5 are available from the authors.


| Entry | Alkyne 1 | Y | Product 3 | Yield (%) b |
|---|---|---|---|---|
| 1 |  | Se |  | 80 |
| 2 |  | Se |  | 63 |
| 3 |  | Se |  | 65 |
| 4 |  | Se |  | 70 |
| 5 |  | Se |  | 60 |
| 6 |  | Se |  | 60 |
| 7 |  | Se |  | 67 |
| 8 |  | Se |  | 52 |
| 9 | 1a | Te |  | 85 |
| 10 | 1b | Te |  | 63 |
| 11 | 1c | Te |  | 55 |
| 12 | 1h | Te |  | 61 |
| 13 |  | Te |  | 63 |
| Entry | Tellanyl Alkyne 3 | Diol 4 | Solubility (mg/mL) b | Yield (%) c |
|---|---|---|---|---|
| 1 |  |  | 2.3 | 50 |
| 2 |  |  | 2.8 | 50 |
| 3 |  |  | 1.5 | 47 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenardão, E.J.; Borges, E.L.; Stach, G.; Soares, L.K.; Alves, D.; Schumacher, R.F.; Bagnoli, L.; Marini, F.; Perin, G. Glycerol as Precursor of Organoselanyl and Organotellanyl Alkynes. Molecules 2017, 22, 391. https://doi.org/10.3390/molecules22030391
Lenardão EJ, Borges EL, Stach G, Soares LK, Alves D, Schumacher RF, Bagnoli L, Marini F, Perin G. Glycerol as Precursor of Organoselanyl and Organotellanyl Alkynes. Molecules. 2017; 22(3):391. https://doi.org/10.3390/molecules22030391
Chicago/Turabian StyleLenardão, Eder J., Elton L. Borges, Guilherme Stach, Liane K. Soares, Diego Alves, Ricardo F. Schumacher, Luana Bagnoli, Francesca Marini, and Gelson Perin. 2017. "Glycerol as Precursor of Organoselanyl and Organotellanyl Alkynes" Molecules 22, no. 3: 391. https://doi.org/10.3390/molecules22030391
APA StyleLenardão, E. J., Borges, E. L., Stach, G., Soares, L. K., Alves, D., Schumacher, R. F., Bagnoli, L., Marini, F., & Perin, G. (2017). Glycerol as Precursor of Organoselanyl and Organotellanyl Alkynes. Molecules, 22(3), 391. https://doi.org/10.3390/molecules22030391