Synthesis and Evaluation of New Oxadiazole, Thiadiazole, and Triazole Derivatives as Potential Anticancer Agents Targeting MMP-9
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Preparation of 5-[(2,4-Dichlorophenyl)amino]-1,3,4-thiadiazole-2(3H)-thione
3.1.2. General Procedure for the Preparation of 4-Amino-5-(phenoxymethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione
3.1.3. General Procedure for the Preparation of 5-[(4-Methoxyphenoxy)methyl]-1,3,4-oxadiazole-2(3H)-thione and 5-{[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]methyl}-1,3,4-oxadiazole-2(3H)-thione
3.1.4. General Procedure for the Preparation of Compounds 1–4
3.1.5. General Procedure for the Preparation of Compounds 5–9
3.2. Cytotoxicity Test
3.3. Matrix Metalloproteinase (MMP) Inhibition Assays
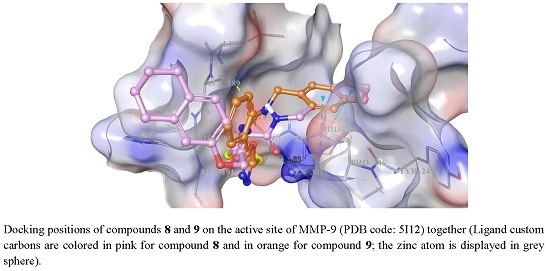
3.4. Docking Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silverstein, A.; Silverstein, V.L.; Silverstein, N. Cancer, Conquering a Deadly Disease; A division of Lerner Publishing Group: Minneapolis, MN, USA, 2006; pp. 11–12. [Google Scholar]
- Almeida, C.A.; Barry, S.A. Cancer: Basic Science and Clinical Aspects; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 2–3. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2016; American Cancer Society: Atlanta, GA, USA, 2016. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chippada-Venkata, U.D.; Oh, W.K. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel) 2014, 6, 1298–1327. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, S.; Ishimaru, N.; Kudo, Y. Matrix metalloproteinases: The gene expression signatures of head and neck cancer progression. Cancers (Basel) 2014, 6, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Eckhardt, S.G. Development of matrix metalloproteinase inhibitors in cancer therapy. J. Natl. Cancer Inst. 2001, 93, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Koh, D.; Kim, G.S.; Lee, S.E.; Noh, H.J.; Kim, S.Y.; Lee, Y.H.; Lim, Y.; Shin, S.Y. 2-Hydroxy-3,4-naphthochalcone (2H-NC) inhibits TNFα-induced tumor invasion through the downregulation of NF-κB-mediated MMP-9 gene expression. Bioorg. Med. Chem. Lett. 2015, 25, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Shon, S.K.; Kim, A.; Kim, J.Y.; Kim, K.I.; Yang, Y.; Lim, J.S. Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells. Biochem. Biophys. Res. Commun. 2009, 385, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Yana, I.; Fujita, C.; Irifune, A.; Takeda, M.; Madachi, A.; Mori, S.; Hamada, Y.; Kawaguchi, N.; Matsuura, N. The role of MT2-MMP in cancer progression. Biochem. Biophys. Res. Commun. 2010, 393, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, Y.; Wang, D.; Gao, H.; Liu, K. MicroRNA-26b suppresses the metastasis of non-small cell lung cancer by targeting MIEN1 via NF-kB/MMP-9/VEGF pathways. Biochem. Biophys. Res. Commun. 2016, 472, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Köhrmann, A.; Kammerer, U.; Kapp, M.; Dietl, J.; Anacker, J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer 2009, 9, 188–208. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.S.; Huang, P.H. The pathobiology of collagens in glioma. Mol. Cancer Res. 2013, 11, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Eide, H.A.; Halvorsen, A.R.; Sandhu, V.; Fåne, A.; Berg, J.; Haakensen, V.D.; Kure, E.H.; Brustugun, O.T.; Kiserud, C.E.; Kyte, J.A.; et al. Non-small cell lung cancer is characterised by a distinct inflammatory signature in serum compared with chronic obstructive pulmonary disease. Clin. Transl. Immunol. 2016, 5, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, N.; Selvamani, A.; Subramanian, R.; Pandi, A.; Thiruvengadam, D. Baicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in vivo. Toxicol. Appl. Pharmacol. 2012, 261, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Sterz, C.M.; Kulle, C.; Dakic, B.; Makarova, G.; Böttcher, M.C.; Bette, M.; Werner, J.A.; Mandic, R. A basal-cell-like compartment in head and neck squamous cell carcinomas represents the invasive front of the tumor and is expressing MMP-9. Oral Oncol. 2010, 46, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Kim, Y.K.; Kim, K.E.; Kim, W.; Park, C.S.; Lee, K.J. Cellular prion protein regulates invasion and migration of breast cancer cells through MMP-9 activity. Biochem. Biophys. Res. Commun. 2016, 470, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.D.; Houghton, A.M. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res. 2011, 71, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chang, L.S. Simvastatin induces NFκB/p65 down-regulation and JNK1/c-Jun/ATF-2 activation, leading to matrix metalloproteinase-9 (MMP-9) but not MMP-2 down-regulation in human leukemia cells. Biochem. Pharmacol. 2014, 92, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Hao, X.; Tang, Y.; Wei, X.; Gong, Y. RABEX-5 overexpression in gastric cancer is correlated with elevated MMP-9 level. Am. J. Transl. Res. 2016, 8, 2365–2374. [Google Scholar] [PubMed]
- Song, T.W.; Lee, J.K.; Lee, S.Y.; Lian, S.; Joo, S.P.; Kim, H.S. Establishment of a malignant model glioma in rats. Nerve 2016, 2, 17–21. [Google Scholar] [CrossRef]
- Passlick, B.; Sienel, W.; Seen-Hibler, R.; Wöckel, W.; Thetter, O.; Mutschler, W.; Pantel, K. Overexpression of matrix metalloproteinase 2 predicts unfavorable outcome in early-stage non-small cell lung cancer. Clin. Cancer Res. 2000, 6, 3944–3948. [Google Scholar] [PubMed]
- El-Badrawy, M.K.; Yousef, A.M.; Shaalan, D.; Elsamanoudy, A.Z. Matrix metalloproteinase-9 expression in lung cancer patients and its relation to serum mmp-9 activity, pathologic type, and prognosis. J. Bronchol. Interv. Pulmonol. 2014, 21, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, T.; Liu, S.; Yoshida, D.; Teramoto, A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol. 2003, 20, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ramanujum, R.; Lin, Y.L.; Liu, J.K.; He, S. Regulatory expression of MMP-8/MMP-9 and inhibition of proliferation, migration and invasion in human lung cancer A549 cells in the presence of HGF variants. Kaohsiung J. Med. Sci. 2013, 29, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Decock, J.; Thirkettle, S.; Wagstaff, L.; Edwards, D.R. Matrix metalloproteinases: Protective roles in cancer. J. Cell. Mol. Med. 2011, 15, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, A.; Fueyo, A.; Folgueras, A.R.; Garabaya, C.; Pennington, C.J.; Pilgrim, S.; Edwards, D.R.; Holliday, D.L.; Jones, J.L.; Span, P.N.; et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008, 68, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Chen, Y.Y.; Liu, P.Y.; Hsu, S.; Sheu, M.J. Pipoxolan inhibits CL1-5 lung cancer cells migration and invasion through inhibition of MMP-9 and MMP-2. Chem. Biol. Interact. 2015, 236, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Väyrynen, J.P.; Vornanen, J.; Tervahartiala, T.; Sorsa, T.; Bloigu, R.; Salo, T.; Tuomisto, A.; Mäkinen, M.J. Serum MMP-8 levels increase in colorectal cancer and correlate with disease course and inflammatory properties of primary tumors. Int. J. Cancer 2012, 131, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, F.; Lin, L.; Fang, C.; Wen, G.; Tsai, T.N.; Pu, X.; Sims, D.; Zhang, Z.; Yin, X.; et al. Functional role of matrix metalloproteinase-8 in stem/progenitor cell migration and their recruitment into atherosclerotic lesions. Circ. Res. 2013, 112, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Patel, G.; Johnson, E.O.; Shah, K. Synthesis and anticancer activities of novel 3,5-disubstituted-1,2,4-oxadiazoles. Bioorg. Med. Chem. Lett. 2009, 19, 2739–2741. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sundaree, S.; Johnson, E.O.; Shah, K. An efficient synthesis and biological study of novel indolyl-1,3,4-oxadiazoles as potent anticancer agents. Bioorg. Med. Chem. Lett. 2009, 19, 4492–4494. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, H.; Yang, Z.M.; Zhu, H.L. Synthesis, molecular modeling and biological evaluation of 2-aminomethyl-5-(quinolin-2-yl)-1,3,4-oxadiazole-2(3H)-thione quinolone derivatives as novel anticancer agent. Eur. J. Med. Chem. 2013, 60, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.B.; Purohit, A.C.; Rajani, D.P.; Moo-Puc, R.; Rivera, G. New 2-benzylsulfanyl-nicotinic acid based 1,3,4-oxadiazoles: Their synthesis and biological evaluation. Eur. J. Med. Chem. 2013, 62, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Alam, M.S.; Hamid, H. 1,3,4-Thiadiazoles: A potent multi targeted pharmacological scaffold. Eur. J. Med. Chem. 2015, 92, 156–177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Vaddula, B.R.; Chang, K.H.; Shah, K. One-pot synthesis and anticancer studies of 2-arylamino-5-aryl-1,3,4-thiadiazoles. Bioorg. Med. Chem. Lett. 2011, 21, 2320–2323. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, M.; Matysiak, J.; Szeliga, M.; Pożarowski, P.; Niewiadomy, A.; Albrecht, J.; Rzeski, W. 2-Amino-1,3,4-thiadiazole derivative (FABT) inhibits the extracellular signal-regulated kinase pathway and induces cell cycle arrest in human non-small lung carcinoma cells. Bioorg. Med. Chem. Lett. 2012, 22, 5466–5469. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. Thiadiazole—A promising structure in medicinal chemistry. ChemMedChem 2013, 8, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Chinthala, Y.; Thakur, S.; Tirunagari, S.; Chinde, S.; Domatti, A.K.; Arigari, N.K.; Srinivas, K.V.N.S.; Alam, S.; Jonnala, K.K.; Khan, F.; et al. Synthesis, docking and ADMET studies of novel chalcone triazoles for anti-cancer and anti-diabetic activity. Eur. J. Med. Chem. 2015, 93, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Kavitha, H.P. Synthesis and biological applications of triazole derivatives—A Review. Mini-Rev. Org. Chem. 2013, 10, 40–65. [Google Scholar] [CrossRef]
- Kaur, R.; Dwivedi, A.R.; Kumar, B.; Kumar, V. Recent developments on 1,2,4-triazole nucleus in anticancer compounds: A review. Anticancer Agents Med. Chem. 2016, 16, 465–489. [Google Scholar] [CrossRef] [PubMed]
- Khorshid, F.A.; Mushref, S.S.; Hefeny, N. An ideal selective anti-cancer agent in vitro: I-Tissue culture study of human lung cancer cells A549. JKAU Med. Sci. 2005, 12, 3–19. [Google Scholar] [CrossRef]
- Nuti, E.; Cuffaro, D.; D’Andrea, F.; Rosalia, L.; Tepshi, L.; Fabbi, M.; Carbotti, G.; Ferrini, S.; Santamaria, S.; Camodeca, C.; et al. Sugar-based arylsulfonamide carboxylates as selective and water-soluble matrix metalloproteinase-12 inhibitors. ChemMedChem 2016, 11, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Madan, A.K.; Bajaj, S.; Dureja, H. Classification models for safe drug molecules. Comput. Toxicol. 2013, 2, 99–124. [Google Scholar]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.W. Tumor metastasis. Matrix metalloproteinase-9 (MMP-9). BioWave 2007, 9, 1–15. [Google Scholar]
- Poudel, B.; Ki, H.H.; Luyen, B.T.; Lee, Y.M.; Kim, Y.H.; Kim, D.K. Triticumoside induces apoptosis via caspase-dependent mitochondrial pathway and inhibits migration through downregulation of MMP2/9 in human lung cancer cells. Acta Biochim. Biophys. Sin. (Shanghai) 2016, 48, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sohn, E.J.; Yoon, S.W.; Kim, C.G.; Lee, S.; Kim, J.Y.; Baek, N.; Kim, S.H. Anti-metastatic effect of dehydrocorydaline on H1299 non-small cell lung carcinoma cells via inhibition of matrix metalloproteinases and B cell lymphoma 2. Phytother. Res. 2017, 31, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.; Cao, J. Selective matrix metalloproteinase (MMP) inhibitors in cancer therapy: Ready for prime time? Cancer Biol. Ther. 2009, 8, 2371–2373. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Löffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Fingleton, B. MMPs as therapeutic targets—Still a viable option? Semin. Cell Dev. Biol. 2008, 19, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ledour, G.; Moroy, G.; Rouffet, M.; Bourguet, E.; Guillaume, D.; Decarme, M.; Elmourabit, H.; Augé, F.; Alix, A.J.; Laronze, J.Y.; et al. Introduction of the 4-(4-bromophenyl)benzenesulfonyl group to hydrazide analogs of Ilomastat leads to potent gelatinase B (MMP-9) inhibitors with improved selectivity. Bioorg. Med. Chem. 2008, 16, 8745–8759. [Google Scholar] [CrossRef] [PubMed]
- Altintop, M.D.; Ozdemir, A.; Kucukoglu, K.; Turan-Zitouni, G.; Nadaroglu, H.; Kaplancikli, Z.A. Synthesis and evaluation of new thiadiazole derivatives as potential inhibitors of human carbonic anhydrase isozymes (hCA-I and hCA-II). J. Enzyme Inhib. Med. Chem. 2015, 30, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Kaplancıklı, Z.A.; Turan-Zitouni, G.; Özdemir, A.; İşcan, G.; Akalın, G.; Ulusoylar Yıldırım, Ş. Synthesis and anticandidal activity of new triazolothiadiazine derivatives. Eur. J. Med. Chem. 2011, 46, 5562–5566. [Google Scholar] [CrossRef] [PubMed]
- Kaplancikli, Z.A.; Altintop, M.D.; Turan-Zitouni, G.; Ozdemir, A.; Ozic, R.; Akalın, G. Synthesis, antimicrobial activity and cytotoxicity of novel oxadiazole derivatives. J. Enzyme Inhib. Med. Chem. 2012, 27, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Özdemir, A.; Turan-Zitouni, G.; Ilgın, S.; Atlı, Ö.; İşcan, G.; Kaplancıklı, Z.A. Synthesis and biological evaluation of some hydrazone derivatives as new anticandidal and anticancer agents. Eur. J. Med. Chem. 2012, 58, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Popiołkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, AP. In vitro toxicity evaluation in the development of new anticancer drugs-genistein glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kaplancıklı, Z.A.; Altıntop, M.D.; Atlı, Ö.; Sever, B.; Baysal, M.; Temel, H.E.; Demirci, F.; Özdemir, A. Synthesis and evaluation of a new series of thiazole derivatives as potential antitumor agents and MMP inhibitors. Anticancer Agents Med. Chem. 2017, 17, 674–681. [Google Scholar] [CrossRef]
- Sever, B.; Altıntop, M.D.; Kuş, G.; Özkurt, M.; Özdemir, A.; Kaplancıklı, Z.A. Indomethacin based new triazolothiadiazine derivatives: Synthesis, evaluation of their anticancer effects on T98 human glioma cell line related to COX-2 inhibition and docking studies. Eur. J. Med. Chem. 2016, 113, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Temel, H.E.; Sever, B.; Akalın Çiftçi, G.; Kaplancıklı, Z.A. Synthesis and evaluation of new benzodioxole-based thiosemicarbazone derivatives as potential antitumor agents. Molecules 2016, 21, 1598. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compounds 1–9 are available from the authors. |





| Compound | IC50 Values for Cell Lines (mM) | SI Values 1 | |||
|---|---|---|---|---|---|
| NIH/3T3 | A549 | C6 | A549 | C6 | |
| 1 | 0.308 ± 0.037 | >1.234 | 0.128 ± 0.027 | 24.959 | 240.625 |
| 2 | 1.413 ± 0.094 | >1.432 | >1.432 | 98.673 | 98.673 |
| 3 | 1.369 ± 0.074 | 1.369 ± 0.058 | 0.507 ± 0.049 | 100 | 270.018 |
| 4 | 1.285 ± 0.090 | >1.285 | >1.285 | 100 | 100 |
| 5 | >1.066 | >1.066 | >1.066 | 100 | 100 |
| 6 | >1.211 | >1.211 | >1.211 | 100 | 100 |
| 7 | 0.100 ± 0.011 | >1.166 | >1.166 | 8.576 | 8.576 |
| 8 | >1.104 | 0.125 ± 0.020 | 0.137 ± 0.015 | 883.200 | 805.839 |
| 9 | 0.888 ± 0.029 | 0.349 ± 0.046 | 0.157 ± 0.016 | 254.441 | 565.605 |
| Cisplatin | >1.166 | 0.140 ± 0.030 | 0.103 ± 0.026 | 1190 | 1617.476 |
| Compound (1.3 μM) | Inhibition % (Mean ± SD) | ||||
|---|---|---|---|---|---|
| MMP-1 | MMP-2 | MMP-8 | MMP-9 | MMP-13 | |
| 1 | ---- | ---- | 15 ± 1.4 | 24.78 ± 0.86 | ---- |
| 8 | ---- | ---- | ---- | 25.75 ± 2.16 | ---- |
| 9 | ---- | ---- | ---- | 30.46 ± 3.27 | ---- |
| NNGH | 87.79 ± 3.49 | 100 | 100 | 88.44 ± 3.35 | 93.74 ± 3.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, A.; Sever, B.; Altıntop, M.D.; Temel, H.E.; Atlı, Ö.; Baysal, M.; Demirci, F. Synthesis and Evaluation of New Oxadiazole, Thiadiazole, and Triazole Derivatives as Potential Anticancer Agents Targeting MMP-9. Molecules 2017, 22, 1109. https://doi.org/10.3390/molecules22071109
Özdemir A, Sever B, Altıntop MD, Temel HE, Atlı Ö, Baysal M, Demirci F. Synthesis and Evaluation of New Oxadiazole, Thiadiazole, and Triazole Derivatives as Potential Anticancer Agents Targeting MMP-9. Molecules. 2017; 22(7):1109. https://doi.org/10.3390/molecules22071109
Chicago/Turabian StyleÖzdemir, Ahmet, Belgin Sever, Mehlika Dilek Altıntop, Halide Edip Temel, Özlem Atlı, Merve Baysal, and Fatih Demirci. 2017. "Synthesis and Evaluation of New Oxadiazole, Thiadiazole, and Triazole Derivatives as Potential Anticancer Agents Targeting MMP-9" Molecules 22, no. 7: 1109. https://doi.org/10.3390/molecules22071109
APA StyleÖzdemir, A., Sever, B., Altıntop, M. D., Temel, H. E., Atlı, Ö., Baysal, M., & Demirci, F. (2017). Synthesis and Evaluation of New Oxadiazole, Thiadiazole, and Triazole Derivatives as Potential Anticancer Agents Targeting MMP-9. Molecules, 22(7), 1109. https://doi.org/10.3390/molecules22071109