Chitosan-Based Nanomaterials for Drug Delivery
Abstract
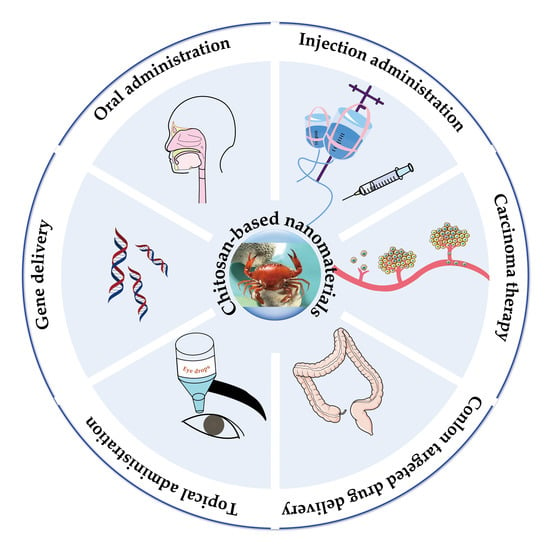
:1. Introduction
2. Approaches in Generating CS Derivatives
2.1. General Modifications of CS to Improve Solubility
2.2. CS-Based Modifications for Targeted Drug Delivery
2.3. CS-Based Modifications to Improve Environmental Responsiveness
2.4. Other Modifications of CS
3. Different Methods of Preparing Nanomaterials Using CS and Its Derivatives
3.1. CS-Based Self-Assembly
3.2. Covalently Crosslinked Nanomaterials
3.3. Ionically Crosslinked Nanomaterials
3.4. Combining CS with Inorganic Nanomaterials
4. Advanced Applications of CS-Based Nanomaterials for Drug Delivery
4.1. Oral Drug Delivery
4.2. Injection Drug Delivery
4.3. Topical Drug Delivery
4.4. Colon-Targeted Drug Delivery
4.5. Carcinoma (Tumors) Therapy
4.6. Gene Delivery
4.7. Vaccine Delivery
5. Conclusions and Future Prospects
Funding
Conflicts of Interest
References
- Fukuta, H.; Goto, T.; Wakami, K.; Ohte, N. Effects of drug and exercise intervention on functional capacity and quality of life in heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Eur. J. Prev. Cardiol. 2016, 23, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.E.; Wasserman, M.A.; Kibbe, M.R. Targeted Nanotherapies for the Treatment of Surgical Diseases. Ann. Surg. 2016, 263, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarian, R.; Herrero, E.P.; Oh, H.; Raghavan, S.R.; Muro, S. Chitosan-Alginate Microcapsules Provide Gastric Protection and Intestinal Release of ICAM-1-Targeting Nanocarriers, Enabling GI Targeting In Vivo. Adv. Funct. Mater. 2016, 26, 3382–3393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.T.; Zhang, Y.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano 2013, 7, 4194–4201. [Google Scholar] [CrossRef] [PubMed]
- Engkagul, V.; Klaharn, I.Y.; Sereemaspun, A.; Chirachanchai, S. Chitosan whisker grafted with oligo(lactic acid) nanoparticles via a green synthesis pathway: Potential as a transdermal drug delivery system. Nanomedicine 2017, 13, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.W.; Jia, Y.Y.; Wan, N.; Luo, M.; Huan, M.L.; Kang, T.B.; Zhou, S.Y.; Zhang, B.L. Design and evaluation of novel pH-sensitive ureido-conjugated chitosan/TPP nanoparticles targeted to Helicobacter pylori. Biomaterials 2016, 84, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona. Sci. Rep. 2017, 7, 10542–10551. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, Y.; Wang, P.; Wang, Y.; Hong, H.; Li, Y.; Qian, J.; Yuan, Y.; Yu, B.; Liu, C. Preferential tumor accumulation and desirable interstitial penetration of poly(lactic-co-glycolic acid) nanoparticles with dual coating of chitosan oligosaccharide and polyethylene glycol-poly(d,l-lactic acid). Acta Biomater. 2016, 29, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Rajitha, P.; Gopinath, D.; Biswas, R.; Sabitha, M.; Jayakumar, R. Chitosan nanoparticles in drug therapy of infectious and inflammatory diseases. Expert Opin. Drug Deliv. 2016, 13, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, H.; Wang, J.; Fang, Q.; Peng, Z. Enzymatically Disulfide Crosslinked Chitosan/Hyaluronic Acid Layer-by-Layer Self-Assembled Microcapsules for Redox-Responsive Controlled Release of Protein. ACS Appl. Mater. Interfaces 2018, 10, 33493–33506. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Burdusel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoanta, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Vasquez Marcano, R.; Tominaga, T.T.; Khalil, N.M.; Pedroso, L.S.; Mainardes, R.M. Chitosan functionalized poly (epsilon-caprolactone) nanoparticles for amphotericin B delivery. Carbohydr. Polym. 2018, 202, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.H.; Mu, Q.; Stephen, Z.R.; Fang, C.; Zhang, M. Hexanoyl-Chitosan-PEG Copolymer Coated Iron Oxide Nanoparticles for Hydrophobic Drug Delivery. ACS Macro Lett. 2015, 4, 403–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhavsar, C.; Momin, M.; Gharat, S.; Omri, A. Functionalized and graft copolymers of chitosan and its pharmaceutical applications. Expert Opin. Drug Deliv. 2017, 14, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, T.; Cao, A.; Sun, J.; Jia, L.; Sheng, R. Morphology-Variable Aggregates Prepared from Cholesterol-Containing Amphiphilic Glycopolymers: Their Protein Recognition/Adsorption and Drug Delivery Applications. Nanomaterials 2018, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Ferji, K.; Venturini, P.; Cleymand, F.; Chassenieux, C.; Six, J.-L. In situ glyco-nanostructure formulation via photo-polymerization induced self-assembly. Polym. Chem. 2018, 9, 2868–2872. [Google Scholar] [CrossRef]
- Sohail, M.F.; Hussain, S.Z.; Saeed, H.; Javed, I.; Sarwar, H.S.; Nadhman, A.; Huma, Z.E.; Rehman, M.; Jahan, S.; Hussain, I.; et al. Polymeric nanocapsules embedded with ultra-small silver nanoclusters for synergistic pharmacology and improved oral delivery of Docetaxel. Sci. Rep. 2018, 8, 13304–13314. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Hoop, M.; Mushtaq, F.; Hurter, C.; Chen, X.Z.; Nelson, B.J.; Pane, S. A smart multifunctional drug delivery nanoplatform for targeting cancer cells. Nanoscale 2016, 8, 12723–12728. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Han, L.; Qin, J.; Ru, G.; Li, R.; Wu, L.; Cui, D.; Yang, P.; He, Y.; Wang, J. N-trimethyl chitosan chloride-coated PLGA nanoparticles overcoming multiple barriers to oral insulin absorption. ACS Appl. Mater. Interfaces 2015, 7, 15430–15441. [Google Scholar] [CrossRef] [PubMed]
- Senel, S.; McClure, S.J. Potential applications of chitosan in veterinary medicine. Adv. Drug Deliv. Rev. 2004, 56, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Motiei, M.; Kashanian, S.; Lucia, L.A.; Khazaei, M. Intrinsic parameters for the synthesis and tuned properties of amphiphilic chitosan drug delivery nanocarriers. J. Control. Release 2017, 260, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, J.; Qiu, Y.; Li, W.; Guo, X.; Li, Q.; Zhang, H.; Zhou, J.; Du, Y.; Yuan, H.; et al. Specific photothermal therapy to the tumors with high EphB4 receptor expression. Biomaterials 2015, 68, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A.; Seguin, C.; Brion, A.; Lavalle, P.; Schaaf, P.; Fournel, S.; Bourel-Bonnet, L.; Frisch, B.; De Giorgi, M. beta-Cyclodextrin-Functionalized Chitosan/Alginate Compact Polyelectrolyte Complexes (CoPECs) as Functional Biomaterials with Anti-Inflammatory Properties. ACS Appl. Mater. Interfaces 2018, 10, 29347–29356. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M. Review paper: Chitosan derivatives as promising materials for controlled drug delivery. J. Biomater. Appl. 2008, 23, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Diebold, Y.; Jarrin, M.; Saez, V.; Carvalho, E.L.; Orea, M.; Calonge, M.; Seijo, B.; Alonso, M.J. Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP). Biomaterials 2007, 28, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Woraphatphadung, T.; Sajomsang, W.; Rojanarata, T.; Ngawhirunpat, T.; Tonglairoum, P.; Opanasopit, P. Development of Chitosan-Based pH-Sensitive Polymeric Micelles Containing Curcumin for Colon-Targeted Drug Delivery. AAPS PharmSciTech 2018, 19, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Xu, F.; Han, Z.; Wei, D.; Jia, D.; Zhou, Y. Tough Magnetic Chitosan Hydrogel Nanocomposites for Remotely Stimulated Drug Release. Biomacromolecules 2018, 19, 3351–3360. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, J.; Pan, H.; He, F.; Liu, Z.; Wu, Q.; Bai, C.; Yu, S.; Yang, X. Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin. Sci. Rep. 2016, 6, 28796–28809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, L.; Li, Q.; Zou, Q.; Du, C. Biomimetic mineralization of carboxymethyl chitosan nanofibers with improved osteogenic activity in vitro and in vivo. Carbohydr. Polym. 2018, 195, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Crayton, S.H.; Thawani, J.P.; Amirshaghaghi, A.; Tsourkas, A.; Cheng, Z. A pH-Responsive Drug-Delivery Platform Based on Glycol Chitosan-Coated Liposomes. Small 2015, 11, 4870–4874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, D.K.; Frisch, S.; Biehl, A.; Terriac, E.; De Rossi, C.; Schwarzkopf, K.; Lautenschlager, F.; Loretz, B.; Murgia, X.; Lehr, C.M. Farnesylated Glycol Chitosan as a Platform for Drug Delivery: Synthesis, Characterization, and Investigation of Mucus-Particle Interactions. Biomacromolecules 2018, 19, 3489–3501. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, M.; Rodriguez-Berna, G.; Gonzalez-Alvarez, I.; Hernandez, M.J.; Corma, A.; Bermejo, M.; Merino, V.; Gonzalez-Alvarez, M. Ionic Hydrogel Based on Chitosan Cross-Linked with 6-Phosphogluconic Trisodium Salt as a Drug Delivery System. Biomacromolecules 2018, 19, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Chen, X.; Gong, H.; Qiu, P.; Xiao, X.; Dang, S.; Hong, A.; Ma, Y. Delivery of a TNF-alpha-derived peptide by nanoparticles enhances its antitumor activity by inducing cell-cycle arrest and caspase-dependent apoptosis. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Chen, Q.; Zhang, Z.; Wang, L.; Denning, T.; Kang, Y.; Merlin, D. TNFalpha gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis. J. Control. Release 2018, 287, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, T.; Yang, Y.; Shao, Z. Colloidal Stability of Silk Fibroin Nanoparticles Coated with Cationic Polymer for Effective Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 21254–21262. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, Y.; Li, Y.; Wu, H.; Yu, F.; Zhou, S.; Xie, L.; Luo, F.; Lin, C.; Hou, Z. Drug/Dye-Loaded, Multifunctional PEG-Chitosan-Iron Oxide Nanocomposites for Methotraxate Synergistically Self-Targeted Cancer Therapy and Dual Model Imaging. ACS Appl. Mater. Interfaces 2015, 7, 11908–11920. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Dobhal, A.; Bangde, P.; Dey, A.; Dandekar, P.; Jain, R. Chitosan-Based Nanoparticulate Systems: Implication Towards Therapeutics Application. In Particulate Technology for Delivery of Therapeutics; Jana, S., Ed.; Springer: Singapore, 2017; pp. 167–225. ISBN 978-981-10-3647-7. [Google Scholar]
- Sun, M.; Li, J.; Zhang, C.; Xie, Y.; Qiao, H.; Su, Z.; Oupicky, D.; Ping, Q. Arginine-Modified Nanostructured Lipid Carriers with Charge-Reversal and pH-Sensitive Membranolytic Properties for Anticancer Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1600693–1600705. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Xie, Z.S.; Xing, L.; Zhang, B.F.; Zhang, J.L.; Cui, P.F.; Qiao, J.B.; Shi, K.; Cho, C.S.; Cho, M.H.; et al. Biocompatible polymeric nanocomplexes as an intracellular stimuli-sensitive prodrug for type-2 diabetes combination therapy. Biomaterials 2015, 73, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Unciti-Broceta, J.D.; Arias, J.L.; Maceira, J.; Soriano, M.; Ortiz-Gonzalez, M.; Hernandez-Quero, J.; Munoz-Torres, M.; de Koning, H.P.; Magez, S.; Garcia-Salcedo, J.A. Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis. PLoS Pathog. 2015, 11, e1004942. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Lopedota, A.; Perrone, M.; Laquintana, V.; Iacobazzi, R.M.; Milella, A.; Fanizza, E.; Depalo, N.; Cutrignelli, A.; Lopalco, A.; et al. Spray-dried mucoadhesives for intravesical drug delivery using N-acetylcysteine- and glutathione-glycol chitosan conjugates. Acta Biomater. 2016, 43, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; He, Z.; Sun, C.; Yang, C.; Zhao, P.; Liu, L.; Leong, K.W.; Mao, H.Q.; Liu, Z.; Chen, Y. Uniform Core-Shell Nanoparticles with Thiolated Hyaluronic Acid Coating to Enhance Oral Delivery of Insulin. Adv. Healthc. Mater. 2018, 7, 1800285–1800296. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, P.; Liang, X.; Gong, X.; Song, T.; Niu, R.; Chang, J. Folate-PEG coated cationic modified chitosan--cholesterol liposomes for tumor-targeted drug delivery. Biomaterials 2010, 31, 4129–4138. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.Y.; Jung, S.; Weitz, D.A.; Yi, H.; Choi, C.H. High-throughput double emulsion-based microfluidic production of hydrogel microspheres with tunable chemical functionalities toward biomolecular conjugation. Lab Chip 2018, 18, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Chowdhuri, A.R.; Singh, T.; Ghosh, S.K.; Sahu, S.K. Carbon Dots Embedded Magnetic Nanoparticles @Chitosan @Metal Organic Framework as a Nanoprobe for pH Sensitive Targeted Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 16573–16583. [Google Scholar] [CrossRef] [PubMed]
- Min, H.S.; You, D.G.; Son, S.; Jeon, S.; Park, J.H.; Lee, S.; Kwon, I.C.; Kim, K. Echogenic Glycol Chitosan Nanoparticles for Ultrasound-Triggered Cancer Theranostics. Theranostics 2015, 5, 1402–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschmann, M.D.; Merzouki, A.; Lavertu, M.; Thibault, M.; Jean, M.; Darras, V. Chitosans for delivery of nucleic acids. Adv. Drug Deliv. Rev. 2013, 65, 1234–1270. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; She, J.; Lin, D.A.; Xu, Y.; Li, X.; Yang, W.F.; Lui, V.W.Y.; Jin, L.; Xie, X.; Su, Y.X. Microneedle-Mediated Delivery of Lipid-Coated Cisplatin Nanoparticles for Efficient and Safe Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 33060–33069. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, Y.; Xiao, Y.; Zhao, Z.; Zhang, C.; Wang, J.; Li, H.; Liu, F.; He, N.; Yuan, Y.; et al. Folate-conjugated nanobubbles selectively target and kill cancer cells via ultrasound-triggered intracellular explosion. Biomaterials 2018, 181, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Yao, Z.; Ding, J.; Min, Q.; Jiang, L.P.; Zhu, J.J. Cascaded Aptamers-Governed Multistage Drug Delivery System Based on Biodegradable Envelope Type Nanovehicle for Targeted Therapy of HER2-overexpressing Breast Cancer. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Kuwako, K.I.; Okano, H. The LKB1-SIK Pathway Controls Dendrite Self-Avoidance in Purkinje Cells. Cell Rep. 2018, 24, 2808–2818. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.P.; Goncalves, R.M.; Antunes, J.C.; Pinto, M.L.; Pinto, A.T.; Castro, F.; Monteiro, C.; Barbosa, M.A.; Oliveira, M.J. An interferon-gamma-delivery system based on chitosan/poly(gamma-glutamic acid) polyelectrolyte complexes modulates macrophage-derived stimulation of cancer cell invasion in vitro. Acta Biomater. 2015, 23, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ji, X.; Yu, B.; Ji, K.; Gallo, D.; Csizmadia, E.; Zhu, M.; Choudhury, M.R.; De La Cruz, L.K.C.; Chittavong, V.; et al. Enrichment-triggered prodrug activation demonstrated through mitochondria-targeted delivery of doxorubicin and carbon monoxide. Nat. Chem. 2018, 10, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chan, J.W.; Moretti, A.; Uhrich, K.E. Designing polymers with sugar-based advantages for bioactive delivery applications. J. Control. Release 2015, 219, 355–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Svirskis, D.; Lu, W.; Ying, M.; Huang, Y.; Wen, J. N-trimethyl chitosan nanoparticles and CSKSSDYQC peptide: N-trimethyl chitosan conjugates enhance the oral bioavailability of gemcitabine to treat breast cancer. J. Control. Release 2018, 277, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles. Acta Biomater. 2017, 47, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Tang, C.; Yin, C. Estrone-modified pH-sensitive glycol chitosan nanoparticles for drug delivery in breast cancer. Acta Biomater. 2018, 73, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, M.I.; Abasolo, I.; Oliva, M.; Andrade, F.; Garcia-Aranda, N.; Melgarejo, M.; Pulido, D.; Corchero, J.L.; Fernandez, Y.; Villaverde, A.; et al. Highly Versatile Polyelectrolyte Complexes for Improving the Enzyme Replacement Therapy of Lysosomal Storage Disorders. ACS Appl. Mater. Interfaces 2016, 8, 25741–25752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugan, C.; Rayappan, K.; Thangam, R.; Bhanumathi, R.; Shanthi, K.; Vivek, R.; Thirumurugan, R.; Bhattacharyya, A.; Sivasubramanian, S.; Gunasekaran, P.; et al. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: An improved nanomedicine strategy. Sci. Rep. 2016, 6, 34053–34064. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.; Ragelle, H.; Pourcelle, V.; Vanvarenberg, K.; Marchand-Brynaert, J.; Preat, V.; Feron, O. Delivery of siRNA targeting tumor metabolism using non-covalent PEGylated chitosan nanoparticles: Identification of an optimal combination of ligand structure, linker and grafting method. J. Control. Release 2016, 223, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Amreddy, N.; Muralidharan, R.; Pathuri, G.; Gali, H.; Chen, A.; Zhao, Y.D.; Munshi, A.; Ramesh, R. Chemodrug delivery using integrin-targeted PLGA-Chitosan nanoparticle for lung cancer therapy. Sci. Rep. 2017, 7, 14674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, J.; He, H.; Han, L.; Qin, J.; Chen, S.; Ru, G.; Li, R.; Yang, P.; Wang, J.; Yang, V.C. Enhancing insulin oral absorption by using mucoadhesive nanoparticles loaded with LMWP-linked insulin conjugates. J. Control. Release 2016, 233, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ramya, A.N.; Joseph, M.M.; Maniganda, S.; Karunakaran, V.; T, T.S.; Maiti, K.K. Emergence of Gold-Mesoporous Silica Hybrid Nanotheranostics: Dox-Encoded, Folate Targeted Chemotherapy with Modulation of SERS Fingerprinting for Apoptosis Toward Tumor Eradication. Small 2017, 13, 1700819–1700834. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chi, T.; Li, T.; Zheng, G.; Fan, L.; Liu, Y.; Chen, X.; Chen, S.; Jia, L.; Shao, J. A smart pH-responsive nano-carrier as a drug delivery system for the targeted delivery of ursolic acid: Suppresses cancer growth and metastasis by modulating P53/MMP-9/PTEN/CD44 mediated multiple signaling pathways. Nanoscale 2017, 9, 9428–9439. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Sharma, S.; Gupta, P.K.; Singh, A.; Teja, B.V.; Dwivedi, P.; Gupta, G.K.; Trivedi, R.; Mishra, P.R. Vitamin B12 functionalized layer by layer calcium phosphate nanoparticles: A mucoadhesive and pH responsive carrier for improved oral delivery of insulin. Acta Biomater. 2016, 31, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, T.; Zheng, G.; Jiang, K.; Fan, L.; Shao, J. Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials 2017, 143, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Scientific ReportsZhu, R.; Zhang, C.G.; Liu, Y.; Yuan, Z.Q.; Chen, W.L.; Yang, S.D.; Li, J.Z.; Zhu, W.J.; Zhou, X.F.; You, B.G.; et al. CD147 monoclonal antibody mediated by chitosan nanoparticles loaded with alpha-hederin enhances antineoplastic activity and cellular uptake in liver cancer cells. Sci. Rep. 2015, 5, 17904–17916. [Google Scholar]
- Vecchies, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Porrelli, D.; Donati, I.; Turco, G.; Paoletti, S.; Marsich, E. Complex Coacervates between a Lactose-Modified Chitosan and Hyaluronic Acid as Radical-Scavenging Drug Carriers. Biomacromolecules 2018. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Tang, C.; Yin, C. Dual-targeting and pH/redox-responsive multi-layered nanocomplexes for smart co-delivery of doxorubicin and siRNA. Biomaterials 2015, 60, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.N.; Zhang, C.N.; Xu, R.; Niu, J.F.; Song, H.J.; Zhang, X.Y.; Wang, W.W.; Wang, Y.M.; Li, C.; Wei, X.Q.; et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gennari, A.; Pelliccia, M.; Donno, R.; Kimber, I.; Tirelli, N. Mannosylation Allows for Synergic (CD44/C-Type Lectin) Uptake of Hyaluronic Acid Nanoparticles in Dendritic Cells, but Only upon Correct Ligand Presentation. Adv. Healthc. Mater. 2016, 5, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Song, Z.; Kim, K.H.; Zheng, N.; Gabrielson, N.P.; Cheng, J. Non-viral gene delivery via membrane-penetrating, mannose-targeting supramolecular self-assembled nanocomplexes. Adv. Mater. 2013, 25, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Chen, Z.R.; Lai, C.H.; Hsieh, C.H.; Feng, C.L. Active Targeted Nanoparticles for Oral Administration of Gastric Cancer Therapy. Biomacromolecules 2015, 16, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, F.; Xiang, K.; Zhang, J.; Xu, M.; Long, P.; Su, H.; Gan, Z.; Yu, Q. CD44-Targeted Facile Enzymatic Activatable Chitosan Nanoparticles for Efficient Antitumor Therapy and Reversal of Multidrug Resistance. Biomacromolecules 2018, 19, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Behroozi, F.; Abdkhodaie, M.J.; Abandansari, H.S.; Satarian, L.; Molazem, M.; Al-Jamal, K.T.; Baharvand, H. Engineering folate-targeting diselenide-containing triblock copolymer as a redox-responsive shell-sheddable micelle for antitumor therapy in vivo. Acta Biomater. 2018, 76, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, W.; Tan, Z.; Zeng, Z.; Yang, H.; Luo, S.; Wang, L.; Xi, T.; Xing, Y. Promoting Immune Efficacy of the Oral Helicobacter pylori Vaccine by HP55/PBCA Nanoparticles against the Gastrointestinal Environment. Mol. Pharm. 2018, 15, 3177–3186. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Hypoxia-/HIF-1alpha-Driven Factors of the Tumor Microenvironment Impeding Antitumor Immune Responses and Promoting Malignant Progression. Adv. Exp. Med. Biol. 2018, 1072, 171–175. [Google Scholar] [PubMed]
- Li, F.; Chen, W.L.; You, B.G.; Liu, Y.; Yang, S.D.; Yuan, Z.Q.; Zhu, W.J.; Li, J.Z.; Qu, C.X.; Zhou, Y.J.; et al. Enhanced Cellular Internalization and On-Demand Intracellular Release of Doxorubicin by Stepwise pH-/Reduction-Responsive Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 32146–32158. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ren, Z.; Chen, M.; Wang, Y.; You, B.; Chen, W.; Qu, C.; Liu, Y.; Zhang, X. Nucleolin-Targeting AS1411-Aptamer-Modified Graft Polymeric Micelle with Dual pH/Redox Sensitivity Designed To Enhance Tumor Therapy through the Codelivery of Doxorubicin/TLR4 siRNA and Suppression of Invasion. Mol. Pharm. 2018, 15, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Ma, Y.; Yu, S.; Ji, C. Smart Multifunctional Magnetic Nanoparticle-Based Drug Delivery System for Cancer Thermo-Chemotherapy and Intracellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 24502–24508. [Google Scholar] [CrossRef] [PubMed]
- Adami, R.C.; Seth, S.; Harvie, P.; Johns, R.; Fam, R.; Fosnaugh, K.; Zhu, T.; Farber, K.; McCutcheon, M.; Goodman, T.T.; et al. An amino acid-based amphoteric liposomal delivery system for systemic administration of siRNA. Mol. Ther. 2011, 19, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Mo, R.; Xue, J.; Zhang, L.; Zhao, Z.; Xue, L.; Ping, Q.; Zhang, C. Sequential intra-intercellular nanoparticle delivery system for deep tumor penetration. Angew. Chem. Int. Ed. Engl. 2014, 53, 6253–6258. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.F.; Lian, Q.; Yang, H.; Wang, X. Surface Molecularly Imprinted Polymer of Chitosan Grafted Poly(methyl methacrylate) for 5-Fluorouracil and Controlled Release. Sci. Rep. 2016, 6, 21409–21420. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Pei, Y.; Hyun, H.; Castanares, M.A.; Collins, D.S.; Yeo, Y. Small molecule delivery to solid tumors with chitosan-coated PLGA particles: A lesson learned from comparative imaging. J. Control. Release 2017, 268, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mukherjee, S.; Yi, J.; Banerjee, P.; Chen, Q.; Zhou, S. Biocompatible Chitosan-Carbon Dot Hybrid Nanogels for NIR-Imaging-Guided Synergistic Photothermal-Chemo Therapy. ACS Appl. Mater. Interfaces 2017, 9, 18639–18649. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, Z.; Zhao, Z.; Miao, Y.; Qiu, Y.; Yang, W.; Jia, X.; Liu, Z.; Hou, H. Design and Development of Graphene Oxide Nanoparticle/Chitosan Hybrids Showing pH-Sensitive Surface Charge-Reversible Ability for Efficient Intracellular Doxorubicin Delivery. ACS Appl. Mater. Interfaces 2018, 10, 6608–6617. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tang, C.; Yin, C. Co-delivery of doxorubicin and interleukin-2 via chitosan based nanoparticles for enhanced antitumor efficacy. Acta Biomater. 2017, 47, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Arora, I.; Javed, K.; Akhtar, M.; Samim, M. Enhancement in the Neuroprotective Power of Riluzole Against Cerebral Ischemia Using a Brain Targeted Drug Delivery Vehicle. ACS Appl. Mater. Interfaces 2016, 8, 19716–19723. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.L.; Kim, J.E.; Im, G.I. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 2016, 39, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pi, J.; Yang, F.; Jiang, J.; Wang, X.; Bai, H.; Shao, M.; Huang, L.; Zhu, H.; Yang, P.; et al. Folate-Chitosan Nanoparticles Loaded with Ursolic Acid Confer Anti-Breast Cancer Activities in vitro and in vivo. Sci. Rep. 2016, 6, 30782–30792. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Termsarasab, U.; Lee, M.Y.; Kim, D.H.; Lee, S.Y.; Kim, J.S.; Cho, H.J.; Kim, D.D. Chemosensitizing indomethacin-conjugated chitosan oligosaccharide nanoparticles for tumor-targeted drug delivery. Acta Biomater. 2017, 57, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnurch, A.; Dunnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Mano, J.F. Chitosan-Based Particles as Controlled Drug Delivery Systems. Drug Deliv. 2008, 12, 41–57. [Google Scholar] [CrossRef]
- Laroui, H.; Dalmasso, G.; Nguyen, H.T.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology 2010, 138, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shan, X.; Zhao, X.; Zha, H.; Chen, X.; Wang, J.; Cai, C.; Wang, X.; Li, G.; Hao, J.; et al. Spongy bilayer dressing composed of chitosan–Ag nanoparticles and chitosan –Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 2017, 157, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Belbekhouche, S.; Mansour, O.; Carbonnier, B. Promising sub-100nm tailor made hollow chitosan/poly(acrylic acid) nanocapsules for antibiotic therapy. J. Colloid. Interface Sci. 2018, 522, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Dozie-Nwachukwu, S.O.; Danyuo, Y.; Obayemi, J.D.; Odusanya, O.S.; Malatesta, K.; Soboyejo, W.O. Extraction and encapsulation of prodigiosin in chitosan microspheres for targeted drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Canepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-alpha. Biomacromolecule 2017, 18, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters influencing the size of chitosan-TPP nano- and microparticles. Sci. Rep. 2018, 8, 4695–4705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, H.; Dong, W.; Zhang, M.; Liu, Q.; Wang, X.; Guan, J.; Wu, H.; Mao, S. Design and intestinal mucus penetration mechanism of core-shell nanocomplex. J. Control. Release 2018, 272, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Mathews, P.D.; Fernandes Patta, A.C.M.; Goncalves, J.V.; Gama, G.D.S.; Garcia, I.T.S.; Mertins, O. Targeted Drug Delivery and Treatment of Endoparasites with Biocompatible Particles of pH-Responsive Structure. Biomacromolecules 2018, 19, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Ju, X.J.; Mu, X.T.; Wang, W.; Xie, R.; Liu, Z.; Chu, L.Y. Core-Shell Chitosan Microcapsules for Programmed Sequential Drug Release. ACS Appl. Mater. Interfaces 2016, 8, 10524–10534. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.Q.; Leite Pereira, C.; Castro, F.; Ferreira, J.R.; Gomez-Lazaro, M.; Aguiar, P.; Barbosa, M.A.; Neidlinger-Wilke, C.; Goncalves, R.M. Anti-inflammatory Chitosan/Poly-gamma-glutamic acid nanoparticles control inflammation while remodeling extracellular matrix in degenerated intervertebral disc. Acta Biomater. 2016, 42, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Vaghari, H.; Anarjan, N.; Ahmadi, O.; Berenjian, A. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2017, 37, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Poudel, B.K.; Ruttala, H.B.; Regmi, S.; Pathak, S.; Gautam, M.; Jin, S.G.; Jeong, J.H.; Choi, H.G.; Kwang Ku, S.; et al. Hyaluronic acid-capped compact silica-supported mesoporous titania nanoparticles for ligand-directed delivery of doxorubicin. Acta Biomater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Nemes, R.; Meszaros, T.; Urbanics, R.; Kok, R.J.; Jackman, J.A.; Cho, N.J.; Storm, G.; Szebeni, J. Complement activation in vitro and reactogenicity of low-molecular weight dextran-coated SPIONs in the pig CARPA model: Correlation with physicochemical features and clinical information. J. Control. Release 2018, 270, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Gurka, M.K.; Pender, D.; Chuong, P.; Fouts, B.L.; Sobelov, A.; McNally, M.W.; Mezera, M.; Woo, S.Y.; McNally, L.R. Identification of pancreatic tumors in vivo with ligand-targeted, pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography. J. Control. Release 2016, 231, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, X.W.; Bao, Y.W.; Chen, Z.; Wu, F.G. Carbon quantum dots with intrinsic mitochondrial targeting ability for mitochondria-based theranostics. Nanoscale 2017, 9, 10948–10960. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, T.; Gimenez-Marques, M.; Bellido, E.; Avila, J.; Asensio, M.C.; Salles, F.; Lozano, M.V.; Guillevic, M.; Simon-Vazquez, R.; Gonzalez-Fernandez, A.; et al. Chitosan-coated mesoporous MIL-100(Fe) nanoparticles as improved bio-compatible oral nanocarriers. Sci. Rep. 2017, 7, 43099–43112. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, J.; Chi, H.; Cao, F. Multifunctional properties of organic-inorganic hybrid nanocomposites based on chitosan derivatives and layered double hydroxides for ocular drug delivery. Acta Biomater. 2016, 36, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Jain, S.; Sau, S.; Iyer, A.K. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: In vitro and ex vivo studies. J. Control. Release 2017, 253, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Belabassi, Y.; Moreau, J.; Gheran, V.; Henoumont, C.; Robert, A.; Callewaert, M.; Rigaux, G.; Cadiou, C.; Vander Elst, L.; Laurent, S.; et al. Synthesis and Characterization of PEGylated and Fluorinated Chitosans: Application to the Synthesis of Targeted Nanoparticles for Drug Delivery. Biomacromolecules 2017, 18, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Huang, Y.; Chen, D.; Qiu, F.; Ma, C.; Jin, X.; Zhu, X.; Zhou, G.; Zhang, Z. pH-Responsive Aerobic Nanoparticles for Effective Photodynamic Therapy. Theranostics 2017, 7, 4537–4550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Wang, J.J.; Tang, X.J.; Du, L.; Li, F. In vitro and in vivo evaluation of functionalized chitosan-Pluronic micelles loaded with myricetin on glioblastoma cancer. Nanomedicine 2016, 12, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, Z.; Luo, Z.; Yu, J.; Liang, R.; Li, X.; Chen, H. Chitosan grafted methoxy poly(ethylene glycol)-poly(epsilon-caprolactone) nanosuspension for ocular delivery of hydrophobic diclofenac. Sci. Rep. 2015, 5, 11337–11348. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.; Valle-Delgado, J.J.; Urban, P.; Baro, E.; Prohens, R.; Mayor, A.; Cistero, P.; Delves, M.; Sinden, R.E.; Grandfils, C.; et al. Adaptation of targeted nanocarriers to changing requirements in antimalarial drug delivery. Nanomedicine 2017, 13, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mei, H.; Gao, Y.; Xie, X.; Nie, H.; Li, T.; Zhang, H.; Jia, L. Co-delivery of oxygen and erlotinib by aptamer-modified liposomal complexes to reverse hypoxia-induced drug resistance in lung cancer. Biomaterials 2017, 145, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Bian, Y.; Liu, Y.; Zhang, X.; Wang, M.; Xing, S.; Li, L.; Gao, D. Combined Near Infrared Photothermal Therapy and Chemotherapy Using Gold Nanoshells Coated Liposomes to Enhance Antitumor Effect. Small 2016, 12, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Crake, C.; Teo, B.; Carugo, D.; de Saint Victor, M.; Seth, A.; Stride, E. Ultrasound-Enhanced siRNA Delivery Using Magnetic Nanoparticle-Loaded Chitosan-Deoxycholic Acid Nanodroplets. Adv. Healthc. Mater. 2017, 6, 1601246–1601254. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Chitosan-modified PLGA nanoparticles tagged with 5TR1 aptamer for in vivo tumor-targeted drug delivery. Cancer Lett. 2017, 400, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Thomas, J.L.; Chen, J.Z.; Jan, J.S.; Lin, H.Y. Activation of tumor suppressor p53 gene expression by magnetic thymine-imprinted chitosan nanoparticles. Chem. Commun. 2016, 52, 2137–2140. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hai, X.; Baigude, H.; Guan, W.; Liu, Z. Fabrication of functional hollow microspheres constructed from MOF shells: Promising drug delivery systems with high loading capacity and targeted transport. Sci. Rep. 2016, 6, 37705–37714. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, G.; Zhang, H.; Hu, S.; Liu, X.; Tang, J.; Shen, Y. The Blood Clearance Kinetics and Pathway of Polymeric Micelles in Cancer Drug Delivery. ACS Nano 2018, 12, 6179–6192. [Google Scholar] [CrossRef] [PubMed]
- De Campos, A.M.; Diebold, Y.; Carvalho, E.L.; Sánchez, A.; Alonso, M.J. Chitosan Nanoparticles as New Ocular Drug Delivery Systems in Vitro Stability in Vivo Fate and Cellular Toxicity. Pharm. Res. 2004, 21, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Mehrotra, R.; Margulis, K.; Zare, R.N. On-demand electrically controlled drug release from resorbable nanocomposite films. Nanoscale 2017, 9, 16429–16436. [Google Scholar] [CrossRef] [PubMed]
- Situ, W.; Xiang, T.; Liang, Y. Chitosan-based particles for protection of proteins during storage and oral administration. Int. J. Biol. Macromol. 2018, 117, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Araujo, F.; Shahbazi, M.A.; Makila, E.; Gomes, M.J.; Airavaara, M.; Kauppinen, E.I.; Raula, J.; Salonen, J.; Hirvonen, J.; et al. Oral hypoglycaemic effect of GLP-1 and DPP4 inhibitor based nanocomposites in a diabetic animal model. J. Control. Release 2016, 232, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Zhu, X.; Shan, W.; Li, L.; Zhong, J.; Zhang, Z.; Huang, Y. Efficient mucus permeation and tight junction opening by dissociable “mucus-inert“ agent coated trimethyl chitosan nanoparticles for oral insulin delivery. J. Control. Release 2016, 222, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Dang, T.; Ma, M.; Tang, B.; Cheng, H.; Jiang, S.; Dong, Y.; Zhang, Y.; Anderson, D. Glucose-Responsive Microgels Integrated with Enzyme Nanocapsules for Closed-Loop Insulin Delivery. ACS Nano 2013, 7, 6758–6766. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Hu, H.; He, Y.; McLeod, H.L.; Xiao, D.; Chen, P.; Shen, L.; Zeng, S.; Yin, X.; Ge, J.; et al. The relationship between miR-302b and EphA2 and their clinical significance in gastric cancer. J. Cancer 2018, 9, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Li, F.; Tang, Y.; Yang, S.D.; Li, J.Z.; Yuan, Z.Q.; Liu, Y.; Zhou, X.F.; Liu, C.; Zhang, X.N. Stepwise pH-responsive nanoparticles for enhanced cellular uptake and on-demand intracellular release of doxorubicin. Int. J. Nanomed. 2017, 12, 4241–4256. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.M.; Lim, S.M.; Choi, S.; Kim, D.H.; Jin, H.E.; Jee, G.; Hong, K.J.; Kim, J.Y. Microneedles: Quick and easy delivery methods of vaccines. Clin. Exp. Vaccine Res. 2017, 6, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Ravina, M.; Paolicelli, P.; Sanchez, A.; Seijo, B.; Alonso, M.J. Chitosan-based nanostructures: A delivery platform for ocular therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Vinay, S.; Ram, G.; Amrita, B.; Sarika, W. Pharmacokinetics and pharmacodynamics of intranasally administered selegiline nanoparticles with improved brain delivery in Parkinson’s disease. Nanomedicine 2018. [Google Scholar] [CrossRef]
- Lupo, N.; Fodor, B.; Muhammad, I.; Yaqoob, M.; Matuszczak, B.; Bernkop-Schnurch, A. Entirely S-protected chitosan: A promising mucoadhesive excipient for metronidazole vaginal tablets. Acta Biomater. 2017, 64, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Katsarov, P.; Pilicheva, B.; Uzunova, Y.; Gergov, G.; Kassarova, M. Chemical cross-linking: A feasible approach to prolong doxylamine/pyridoxine release from spray-dried chitosan microspheres. Eur. J. Pharm. Sci. 2018, 123, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.A.; Naeem, M.; Bae, J.; Kim, J.; Lee, J.; Hasan, N.; Kim, W.; Im, E.; Jung, Y.; Yoo, J.W. Colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit S multilayers for the treatment of inflammatory bowel disease. Carbohydr. Polym. 2018, 198, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wu, W.-R.; Qin, Y.-F.; Hu, J.; Liu, C.; Seeberger, P.H.; Yin, J. An Integrated Therapeutic Delivery System for Enhanced Treatment of Hepatocellular Carcinoma. Adv. Funct. Mater. 2018, 28, 1706600. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Gadora, K.; Cheng, H.; Peng, J.; Ma, Y.; Guo, Y.; Chi, C.; Zhou, J.; Ding, Y. Dual-sensitive chitosan derivative micelles for site-specific drug release in the treatment of chicken coccidiosis. RSC Adv. 2018, 8, 14515–14526. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Huang, R.; Li, X.; Liu, S.; Shen, Y.M. Controllable release of nitric oxide and doxorubicin from engineered nanospheres for synergistic tumor therapy. Acta Biomater. 2017, 57, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Majidi, S.; Zangabad, P.S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Chitosan-based multifunctional nanomedicines and theranostics for targeted therapy of cancer. Med. Res. Rev. 2018, 38, 2110–2136. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Cilurzo, F.; Maiuolo, J.; Federico, C.; Di Martino, M.T.; Cristiano, M.C.; Tassone, P.; Fresta, M.; Paolino, D. Delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci. Rep. 2015, 5, 17579–17589. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.W.; Li, J.; Zhao, Z.; Zhang, L.; Peng, G.; Liang, L. Molecular dynamics study on the mechanism of polynucleotide encapsulation by chitosan. Sci. Rep. 2017, 7, 5050–5058. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wang, X.; Jin, Y.; Hu, W.; Duan, Y.; Shi, A.; Du, Y.; Song, D.; Yang, M.; Li, S.; et al. Nogo-B receptor increases the resistance to tamoxifen in estrogen receptor-positive breast cancer cells. Breast Cancer Res. 2018, 20, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Zhang, X.; Zhao, W.; Chen, X. Psoralidin induces autophagy through ROS generation which inhibits the proliferation of human lung cancer A549 cells. PeerJ 2014, 2, e555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Park, J.S.; Lee, S.J.; Jang, J.; Park, J.S.; Back, S.H.; Bahn, G.; Park, J.H.; Kang, Y.M.; Kim, S.H.; et al. Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy. J. Control. Release 2015, 216, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Han, H.D.; Byeon, Y.; Jang, J.H.; Jeon, H.N.; Kim, G.H.; Kim, M.G.; Pack, C.G.; Kang, T.H.; Jung, I.D.; Lim, Y.T.; et al. In vivo stepwise immunomodulation using chitosan nanoparticles as a platform nanotechnology for cancer immunotherapy. Sci. Rep. 2016, 6, 38348–38360. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Jenjob, R.; Phakkeeree, T.; Crespy, D. Saccharides, oligosaccharides, and polysaccharides nanoparticles for biomedical applications. J. Control. Release 2018, 284, 188–212. [Google Scholar] [CrossRef] [PubMed]



| Reaction Type | Chitosan Derivatives | |||
|---|---|---|---|---|
 N-alkylation |  |  |  | |
| Trimethyl chitosan [23] N-(2-hydroxy)-propyl-3-trimethyl chitosan [49] N-octyl chitosan [45] | ||||
 |  | |||
| Octadecyl quaternization chitosan [50] | N-carboxymethyl chitosan [51] | |||
 N-acylation |  |  | ||
| N-succinyl chitosan [31] | N-acetylcysteine chitosan [33] | |||
 O-alkylation |  |  | ||
| O-carboxymethyl chitosan [52] | Glycol chitosan [53] | |||
| Ligand Type | Ligand | Receptor/Targeting Site | Ref. |
|---|---|---|---|
| Peptide/protein | CSKSSDYQC peptide | Goblet cells | [63] |
| EGFR-specific peptide | Asialoglycoprotein receptor | [64] | |
| Arginine-glycine-aspartic acid | Integrin αvβ3 | [66,67,68,69] | |
| TNYLFSPNGPIARAW peptide | EphB4 | [26] | |
| Cell penetrating peptide | Intestinal mucosal layer | [70] | |
| Vitamin | Folate (FA) | Folate receptor | [71,72] |
| Vitamin B12 | Epithelial cells | [73] | |
| Hormone | Estrone | Breast cancer tissues | [65] |
| Carbohydrate | Lactobionic acid | ASGPR | [74] |
| Lactose | ASGPR | [75,76] | |
| Galactose | ASGPR | [77] | |
| Mannose | Mannose receptor | [78,79,80] | |
| Fucose | Fucosylated abnormal cell | [81] | |
| Hyaluronic acid (HA) | CD44 | [82] |
| Triggering Conditions | Function | Grafting Molecule | Ref. |
|---|---|---|---|
| pH | Adjust the isoelectric point of the chitosan derivative to the expected point and reverse the charge under a certain pH condition. Simultaneously, keep the drug delivery system stable in the body fluid circulation and release the drug in response to the pH of the specific sites. | Arginine | [45] |
| Lysine | [50] | ||
| Vitamin B12 | [73] | ||
| Succinylation | [90,92] | ||
| Gly-Phe-Leu-Gly (GFLG) tetrapeptide | [82] | ||
| Ethylenediaminetetraacetic acid (EDTA) | [93] | ||
| Dimethylmaleic anhydride (DMMA) | [94] | ||
| Cis-aconitic anhydride (CA) | [95] | ||
| Poly(2-(diisopropylamino)ethyl methacrylate) | [65] | ||
| Poly(methyl methacrylate) | [91] | ||
| Polyacrylic acid (PAA) | [96] | ||
| Temperature | Act as a hydrophobic core and release drug with temperature responsive. | pNIPAA | [88,90] |
| Pluronics | [97] | ||
| Enzyme | Controlled drug release via structural collapse caused by enzymatic degradation. | GFLG peptide | [82] |
| Magnetic field | Direct delivery of the nanoparticle to specific site though external magnetic field. | Fe3O4 | [88] |
| Morphology | The Role Chitosan Played | Preparation Method | Application | Ref |
|---|---|---|---|---|
| Nanogels | Chitosan-carbon dot hybrid nanogels | Covalent cross-linking | Photothermal−chemo therapy | [93] |
| pH responsive eucalyptus oil coated double walled biodegradable nanogels | Ion crosslinking | Controlled drug delivery | [119] | |
| PEGylated and fluorinated chitosan nanogel | Covalent modification | Targeted drug delivery | [120] | |
| Reversible swelling-shrinking nanogel | Covalent modification/cross-linking | Character of deep tumor penetration | [90] | |
| Micelles | Chitosan-based pH-sensitive polymeric micelles | Covalent modification/self-assembly | Colon-targeted drug delivery | [31] |
| pH-responsive aerobic micelles | Ion crosslinking | Photodynamic therapy | [121] | |
| Chitosan-pluronic micelles | Covalent modification/self-assembly | Drug delivery for glioblastoma cancer | [122] | |
| Multifunctional nanoparticles | Covalent modification/self-assembly | Targeted photothermal therapy | [26] | |
| Chitosan grafted MPEG-PCL | Covalent modification/self-assembly | Ocular delivery of hydrophobic drug | [123] | |
| Nanofibers | Biomimetic mineralization of carboxymethyl chitosan nanofibers | Electrospinning process | Improve osteogenic activity | [34] |
| Liposomes | Arginine-modified nanostructured lipid carriers | Covalent modification/self-assembly | Anticancer drug delivery | [45] |
| Glycosaminoglycan modified chitosan liposome | Covalent modification | Antimalarial drug delivery | [124] | |
| Aptamer-modified liposomal complexes | Covalent modification/other processing | Reverse drug resistance in lung cancer | [125] | |
| Gold nanoshell-coated liposomes | Covalent modification/electrostatic adsorption | Photothermal and chemotherapy | [126] | |
| Glycol chitosan-coated liposomes | Covalent modification/self-assembly | pH-responsive drug-delivery | [35] | |
| Nanosphere | Magnetic nanoparticle-loaded chitosan-deoxycholic acid nanodroplets | Covalent modification, self-assembly | siRNA Delivery | [127] |
| Smart pH-responsive nanocarrier | Covalent modification/electrostatic adsorption | Targeted delivery of ursolic acid | [72] | |
| Thermoresponsive nanospheres | Covalent modification/emulsification/solvent evaporation method | Release drug for the treatment of osteoarthritis | [97] | |
| Nano-particles | Uniform core-shell nanoparticles | Ion crosslinking | Enhance oral delivery of insulin | [49] |
| N-trimethyl chitosan nanoparticles | Covalent modification/self-assembly | Oral delivery to treat breast cancer | [63] | |
| Chitosan-modified PLGA nanoparticles | Ion crosslinking | Tumor-targeted drug delivery | [128] | |
| EGFR-targeted chitosan nanoparticles | Covalent modification/self-assembly | SiRNA delivery | [64] | |
| Indomethacin-conjugated chitosan oligosaccharide nanoparticle | Covalent modification/self-assembly | Prodrug and tumor-targeted drug delivery | [99] | |
| Inorganic nano-materials | Viable smart targeted nanoenvelope delivery system | Covalent modification/self-assembly | Dox encapsulated and targeted therapy | [71] |
| Multifunctional magnetic nanoparticles | Covalent modification/ sonication treatment | Thermo-Chemotherapy Intracellular Imaging | [88] | |
| Combinatorial nanocarrier | Covalent modification/ion crosslinking | Drug delivery for breast cancer | [67] | |
| Magnetic thymine-imprinted chitosan nanoparticles | Physical adsorption | Gene therapy | [129] | |
| Functional hollow microspheres constructed from MOF shells | Covalent modification/Physical adsorption | Drug delivery and targeted transport | [130] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. https://doi.org/10.3390/molecules23102661
Li J, Cai C, Li J, Li J, Li J, Sun T, Wang L, Wu H, Yu G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules. 2018; 23(10):2661. https://doi.org/10.3390/molecules23102661
Chicago/Turabian StyleLi, Jianghua, Chao Cai, Jiarui Li, Jun Li, Jia Li, Tiantian Sun, Lihao Wang, Haotian Wu, and Guangli Yu. 2018. "Chitosan-Based Nanomaterials for Drug Delivery" Molecules 23, no. 10: 2661. https://doi.org/10.3390/molecules23102661
APA StyleLi, J., Cai, C., Li, J., Li, J., Li, J., Sun, T., Wang, L., Wu, H., & Yu, G. (2018). Chitosan-Based Nanomaterials for Drug Delivery. Molecules, 23(10), 2661. https://doi.org/10.3390/molecules23102661