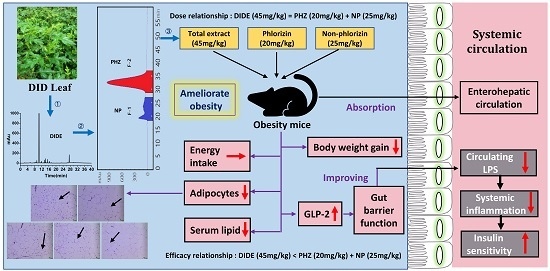
Purified Phlorizin from DocynIa Indica (Wall.) Decne by HSCCC, Compared with Whole Extract, Phlorizin and Non-Phlorizin Fragment Ameliorate Obesity, Insulin Resistance, and Improves Intestinal Barrier Function in High-Fat-Diet-Fed Mice
Abstract
:1. Introduction
2. Results
2.1. Selection of the Two-Phase Solvent System and Other Conditions of HSCCC
2.2. HSCCC Separation Procedure
2.3. Effects of DIDE, PHZ and NP on Body Weight, Fat Index, and Fat Cells
2.4. Effects of DIDE, PHZ and NP on Serum Lipid Levels
2.5. DIDE, PHZ and NP Improves Diet-Induced Insulin Resistance
2.6. DIDE, PHZ, and NP Ameliorated HFD-Induced Inflammation Cytokines Levels in Serum
2.7. DIDE, PHZ and NP Improve Intestinal Barrier Damage and Reduce LPS Level in the Blood
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of DIDE Powder
4.3. Selection of Two-Phase Solvent System
4.4. HSCCC Separation Procedure
4.5. Determination of Compounds by HPLC
4.6. Animals and Diets
4.7. Biochemical Analysis
4.8. Histopathological Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DID | Docynia indica (Wall.) Decne |
| PHZ | phlorizin |
| NP | non-phlorizin |
| DIDE | extract |
| HSCCC | high-speed counter-current chromatography |
| HFD | high-fat diet |
| NCD | normal chow diet |
| LPS | lipopolysaccharides |
| GLP-2 | glucagon-like peptide-2 |
| SGLTs | sodium−glucose symporters |
| TC | total-cholesterol |
| TG | total-cholesterol |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| TNF-α | tumor necrosis factor |
| MCP-1 | monocyte chemoattractant protein-1 |
| IL-6 | interleukin-6 |
| IL-10 | interleukin-10 |
| HOMA | homeostasis model assessment |
| HOMA-IR | insulin resistance index |
| HOMA-IS | insulin sensitive index |
| BW | body weight |
References
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Patell, R.; Dosi, R.; Joshi, H.; Sheth, S.; Shah, P.; Jasdanwala, S. Non-alcoholic fatty liver disease (NAFLD) in obesity. J. Clin. Diagn. Res. 2014, 8, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Eckel, R.H. Obesity and cardiovascular disease. Curr. Atheroscler. Rep. 2002, 4, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.J.L. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Powell, T.M.; Khera, A. Therapeutic approaches to obesity. Curr. Treat. Options Cardiovasc. Med. 2010, 12, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin. Drug Saf. 2012, 11, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Zhou, K. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J. Agric. Food Chem. 2010, 58, 11250–11256. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Ko, C.H.; Ma, N.; Tan, P.W.; Fu, W.M.; He, J.Y. Chemical profiles, antioxidant and anti-obesity effects of extract of Bambusa textilis McClure leaves. J. Funct. Foods 2016, 22, 533–546. [Google Scholar] [CrossRef]
- Chinese Flora Editorial Board of the Chinese Academy of Sciences Flora of China; Science Press: Beijing, China, 2003; Volume 9, pp. 170–171.
- Yuan, W.; Li, W.X.; Lin, Q.; Yang, Z.S. Studies on resource and utilization of Docynia in yunnan. J. Yunnan Agric. Univ. (Nat. Sci.) 1995, 10, 256–258. [Google Scholar]
- Deng, X.; Zhao, X.; Lan, Z.; Jiang, J.; Yin, W.; Chen, L. Anti-tumor effects of flavonoids from the ethnic medicine Docynia delavayi (Franch.) Schneid. and its possible mechanism. J. Med. Food 2014, 17, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shu, G.; Chen, L.; Mi, X.; Mei, Z.; Deng, X. A flavonoid component from Docynia delavayi (Franch.) Schneid represses transplanted H22 hepatoma growth and exhibits low toxic effect on tumor-bearing mice. Food Chem. Toxicol. 2012, 50, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Yi, Z.; Zhao, Y.H. Chinese Dai Medicine Colorful Illustrations. In Chinese Dai Medicine Colorful Illustrations; Yunnan National Press: Kunming, China, 2003; p. 380. [Google Scholar]
- Loan, N.T.T.; Tan, H.T.M.; Tam, V.T.H.; Luan, C.L.; Huong, L.M.; Lien, D.N. Anti-obesity and body weight reducing effect of Docynia indica (Wall.) Decne fruit extract fractions in experimentally obese mice. VNU J. Sci. Nat. Sci. Technol. 2011, 27, 125–133. [Google Scholar]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.; Xu, J.; Zhou, Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mei, X.; Wang, Z.; Wu, J.; Liu, G.; Hu, H.; Li, Q. Chemical Fingerprint and Quantitative Analysis for the Quality Evaluation of Docynia dcne Leaves by High-Performance Liquid Chromatography Coupled with Chemometrics Analysis. J. Chromatogr. Sci. 2018, 56, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Liu, G.; Zhang, X.Y.; Zhu, M.J.; Peng, T.; Wang, Z.G. Determination of polyphenol contents in Docynia Dcne. Food Sci. 2014, 35, 295–300. [Google Scholar]
- Ehrenkranz, J.R.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Najafian, M.; Jahromi, M.Z.; Nowroznejhad, M.J.; Khajeaian, P.; Kargar, M.M.; Sadeghi, M.; Arasteh, A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2012, 39, 5299–5306. [Google Scholar] [CrossRef] [PubMed]
- Su-Kyung, S.; Su-Jung, C.; Ju, J.U.; Ri, R.; Myung-Sook, C. Phlorizin supplementation attenuates obesity, inflammation, and hyperglycemia in diet-induced obese mice fed a high-fat diet. Nutrients 2016, 8, 92. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Kipletting, T.E.; Han, L.; Jia, Y.; Zhang, L.; Wang, Y.; Zhang, X.; Zhang, Y. Fishing and knockout of bioactive compounds using a combination of high-speed counter-current chromatography (HSCCC) and preparative HPLC for evaluating the holistic efficacy and interaction of the components of Herba Epimedii. J. Ethnopharmacol. 2013, 147, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Ruiz-Angel, M.J.; Carda-Broch, S. Countercurrent chromatography: People and applications. J. Chromatogr. A 2009, 1216, 4206–4217. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, C.; Pei, H.; Sun, B. Separation and identification of polyphenols in apple pomace by high-speed counter-current chromatography and high-performance liquid chromatography coupled with mass spectrometry. J. Chromatogr. A 2009, 1216, 4268–4274. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, H.; Yan, X.; Zhu, P.; Chen, J.; Yang, R. Preparative isolation and purification of macrolactin antibiotics from marine bacterium Bacillus amyloliquefaciens using high-speed counter-current chromatography in stepwise elution mode. J. Chromatogr. A 2013, 1272, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. J. Clin. Otolaryngol. Head Neck Surg. 2018, 359, eaar3318. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, W.; Liu, Z. Preparative isolation, quantification and antioxidant activity of dihydrochalcones from Sweet Tea (Lithocarpus polystachyus Rehd.). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1002, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Sakar, Y.; Lepage, P.; Devime, F.; Langelier, B.; Doré, J.; Covasa, M. Replication of obesity and associated signaling pathways through transfer of microbiota from obese-prone rats. Diabetes 2015, 63, 1624. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M. Lipids and diabetes: A fatal combination? Diabet. Med. 1998, 15, 359. [Google Scholar] [CrossRef]
- Emerging Risk Factors, C.; Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.; Duckworth, C.A. Gut microbiota control gut permeability through GLP-2. Gastroenterology 2010, 138, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Citi, S. Intestinal barriers protect against disease. Science 2018, 359, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Beck, P.L.; McKay, D.M. Neuroimmunophysiology of the gut: Advances and emerging concepts focusing on the epithelium. Nat. Rev. Gastroenterol. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Xiao, X.H.; Wang, J.B.; Jian, W. Consistency of efficacy-equivalent: Key essential point of quality control for Chinese materia medica. Chin. Tradit. Herb. Drugs 2015, 46, 1571–1575. [Google Scholar] [CrossRef]
- Zhou, C.J.; Huang, S.; Liu, J.Q.; Qiu, S.Q.; Xie, F.Y.; Song, H.P.; Li, Y.S.; Hou, S.Z.; Lai, X.P. Sweet tea leaves extract improves leptin resistance in diet-induced obese rats. J. Ethnopharmacol. 2013, 145, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.Y.; Liu, G.; Wang, Z.G.; Liu, H.X. Study on polyphenol purification by macroporous resin and inhibition activity to pancreatic amylase of polyphenol from Docyniaindica (Wall.) Dcne leaves. Sci. Technol. Food Ind. 2016, 37, 143–148. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, W.; Gu, Y. Online isolation and purification of four phthalide compounds from Chuanxiong rhizoma using high-speed counter-current chromatography coupled with semi-preparative liquid chromatography. J. Chromatogr. A 2013, 1284, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.J.; Liu, G.; Mei, X.R.; Hu, T.T.; Du, J.; Zhang, X.Y. Effect of solid-state fermentation with eurotium cristatum on the quality and chemical compositions of Docynia indica tea. J. Food Sci. Biotechnol. 2017, 36, 834–842. [Google Scholar] [CrossRef]
- Mei, X.; Zhang, X.; Wang, Z.; Gao, Z.; Liu, G.; Hu, H.; Zou, L.; Li, X. Insulin Sensitivity-Enhancing Activity of Phlorizin Is Associated with Lipopolysaccharide Decrease and Gut Microbiota Changes in Obese and Type 2 Diabetes (db/db) Mice. J. Agric. Food Chem. 2016, 64, 7502–7511. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Li, B.; Yu, F.; Lu, W.; Zhang, Z.; Yin, M.; Gao, H. Investigation of the Protective Effects of Phlorizin on Diabetic Cardiomyopathy in db/db Mice by Quantitative Proteomics. J. Diabetes Res. 2013, 2013, 263845. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Qiang, C.; Xu, D.; Yang, X.; Zheng, X. Purified Betacyanins from Hylocereus undatus Peel Ameliorate Obesity and Insulin Resistance in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2015, 64, 236–244. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Docynia indica (Wall.) Decne, phlorizin and no- phlorizin are available from the authors. |






| Solvent System | Ratio(v/v) | K |
|---|---|---|
| Ethyl acetate-methanol-water | 4:1:5 | 4.5 |
| Ethyl acetate-methanol-water | 4:2:5 | 12 |
| N-butanol-ethyl acetate-methanol-water | 1:1:0.5:2 | 5.3 |
| N-hexane-ethyl acetate-methanol-water | 2:3:1:5 | 0.1 |
| N-hexane-ethyl acetate-methanol-water | 1:3:2:4 | 0.2 |
| N-hexane-ethyl acetate-methanol-water | 1:4:1:5 | 1.2 |
| N-hexane-ethyl acetate-methanol-water | 1:6:3:6 | 2.5 |
| Parameter | NCD (n = 10) | HFD (n = 10) | HFD + DIDE (n = 10) | HFD+PHZ (n = 10) | HFD + NP (n = 10) |
|---|---|---|---|---|---|
| Body Weight Measurements | |||||
| Body weight gain (g) | 4.01 ± 0.38 | 14.78 ± 2.07 &&& | 7.74 ± 1.05 *** | 8.19 ± 0.59 ** | 8.2 ± 1.01 ** |
| Body weight gain rate (%) | 16.79 | 54.99 | 32.13 | 33.84 | 34.87 |
| Relative body weight gain rate (%) | 0 | 38.2 | 15.35 | 17.05 | 18.08 |
| Food intake (g/day) | 2.29 ± 0.15 | 2.33 ± 0.10 | 2.3 ± 0.33 | 2.36 ± 0.03 | 2.19 ± 0.06 |
| Energy intake (kcal/day) | 5.67 ± 0.38 | 11.01 ± 0.46 &&& | 10.87 ± 1.57 | 11.14 ± 0.14 | 10.35 ± 0.27 |
| Serum Biochemical Variables | |||||
| TC (mmol/L) | 5.91 ± 0.33 | 9.24 ± 0.39 && | 6.33 ± 0.11 ** | 6.49 ± 0.06 ** | 6.81 ± 0.69 ** |
| TG (mmol/L) | 24.15 ± 1.38 | 36.89 ± 1.92 &&& | 26.08 ± 1.75 ** | 26.40 ± 1.56 ** | 30.59 ± 3.74 |
| HDL (mmol/L) | 144.26 ± 4.85 | 86.60 ± 0.62 &&& | 121.24 ± 5.51 *** | 132.32 ± 3.30 *** | 133.17 ± 6.75 *** |
| LDL (mmol/L) | 139.46 ± 2.18 | 196.05 ± 4.54 &&& | 151.72 ± 5.45 *** | 127.90 ± 4.22 *** | 152.66 ± 4.08 *** |
| Insulin (nIU/mL) | 6.59 ± 0.33 | 9.64 ± 0.28 &&& | 6.87 ± 0.52 *** | 7.04 ± 0.19 *** | 6.69 ± 0.07 *** |
| Serum TNF-α (pg/mL) | 0.70 ± 0.03 | 0.90 ± 0.01 &&& | 0.67 ± 0.02 *** | 0.71 ± 0.01 *** | 0.81 ± 0.01 *** |
| Serum MCP-1 (pg/mL) | 297.38 ± 13.69 | 431.18 ± 5.78 &&& | 280.53 ± 5.78 *** | 318.72 ± 9.58 ** | 353.48 ± 35.01 |
| Serum IL-6 (pg/mL) | 115.21 ± 9.98 | 144.55 ± 6.15 && | 115.21 ± 9.98 * | 105.43 ± 6.35 ** | 105.78 ± 8.30 ** |
| Serum IL-10 (pg/mL) | 1.07 ± 0.04 | 0.80 ± 0.03 & | 1.08 ± 0.10 * | 1.07 ± 0.06 * | 1.06 ± 0.02 |
| Serum LPS (pg/mL) | 14.39 ± 0.35 | 17.82 ± 0.88 &&& | 13.54 ± 0.09 *** | 13.35 ± 0.31 *** | 12.82 ± 0.43 *** |
| Serum GLP-2 (pg/mL) | 11.73 ± 0.15 | 9.33 ± 0.31 &&& | 11.77 ± 0.40 ** | 12.26 ± 0.41 ** | 11.85 ± 0.45 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-y.; Yi, K.; Chen, J.; Li, R.-p.; Xie, J.; Jin, Y.; Mei, X.-r.; Li, Y.-j.; Liu, G.; Wang, Z.-g. Purified Phlorizin from DocynIa Indica (Wall.) Decne by HSCCC, Compared with Whole Extract, Phlorizin and Non-Phlorizin Fragment Ameliorate Obesity, Insulin Resistance, and Improves Intestinal Barrier Function in High-Fat-Diet-Fed Mice. Molecules 2018, 23, 2701. https://doi.org/10.3390/molecules23102701
Zhang X-y, Yi K, Chen J, Li R-p, Xie J, Jin Y, Mei X-r, Li Y-j, Liu G, Wang Z-g. Purified Phlorizin from DocynIa Indica (Wall.) Decne by HSCCC, Compared with Whole Extract, Phlorizin and Non-Phlorizin Fragment Ameliorate Obesity, Insulin Resistance, and Improves Intestinal Barrier Function in High-Fat-Diet-Fed Mice. Molecules. 2018; 23(10):2701. https://doi.org/10.3390/molecules23102701
Chicago/Turabian StyleZhang, Xiao-yu, Kang Yi, Jiang Chen, Rui-ping Li, Jie Xie, Yan Jin, Xue-ran Mei, Yao-jun Li, Gang Liu, and Zhan-guo Wang. 2018. "Purified Phlorizin from DocynIa Indica (Wall.) Decne by HSCCC, Compared with Whole Extract, Phlorizin and Non-Phlorizin Fragment Ameliorate Obesity, Insulin Resistance, and Improves Intestinal Barrier Function in High-Fat-Diet-Fed Mice" Molecules 23, no. 10: 2701. https://doi.org/10.3390/molecules23102701
APA StyleZhang, X.-y., Yi, K., Chen, J., Li, R.-p., Xie, J., Jin, Y., Mei, X.-r., Li, Y.-j., Liu, G., & Wang, Z.-g. (2018). Purified Phlorizin from DocynIa Indica (Wall.) Decne by HSCCC, Compared with Whole Extract, Phlorizin and Non-Phlorizin Fragment Ameliorate Obesity, Insulin Resistance, and Improves Intestinal Barrier Function in High-Fat-Diet-Fed Mice. Molecules, 23(10), 2701. https://doi.org/10.3390/molecules23102701