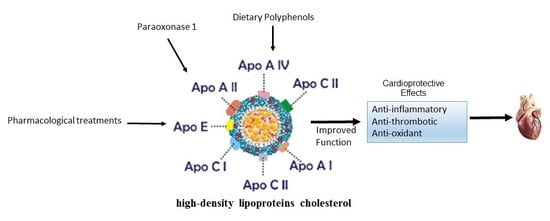
Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease
Abstract
:1. Lipoproteins: Classes and Functions
1.1. HDL Physiological Importance and Metabolism
1.2. Association of Inflammation and HDL in the Development of Cardiovascular Disease
1.3. Inflammation and Antioxidants
1.4. Anti-Thrombotic
1.5. HDL Dysfunctional
1.6. Pharmacological therapies
1.7. Recent Strategies for Improving HDL Function
1.8. Endothelial Dysfunction and Exogenous Antioxidants
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahley, R.W.; Innerarity, T.L.; Rall, S.C.; Weisgraber, K.H. Plasma lipoproteins: Apolipoprotein structure and function. J. Lipid Res. 1984, 25, 1277–1294. [Google Scholar] [PubMed]
- Mahley, R.W.; Ji, Z.S. Remnant lipoprotein metabolism: Key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J. Lipid Res. 1999, 40, 1–16. [Google Scholar] [PubMed]
- Segrest, J.P.; Jones, M.K.; De Loof, H.; Dashti, N. Structure of apolipoprotein B-100 in low density lipoproteins. J. Lipid Res. 2001, 42, 1346–1367. [Google Scholar] [PubMed]
- Green, P.H.; Glickman, R.M.; Riley, J.W.; Quinet, E. Human apolipoprotein A-IV. Intestinal origin and distribution in plasma. J. Clin. Investig. 1980, 65, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Krauss, R.M.; Ma, J.; Blanche, P.J.; Holl, L.G.; Sacks, F.M.; Hennekens, C.H. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA 1996, 276, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Posadas-Romero, C.; Posadas-Sánchez, R.; Juárez-Rojas, J.G.; Medina-Urrutia, A.; Jorge-Galarza, E.; Cardoso-Saldaña, G.; Caracas-Portilla, N.; Mendoza-Peŕez, E. High and low density lipoprotein abnormalities in coronary patients with LDL-C at target and uncontrolled HDL-C and triglycerides. Arch. Cardiol. Mex. 2008, 78, 30–39. [Google Scholar] [PubMed]
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Skene, A.M.; et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004, 350, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- LaRosa, J.C.; Deedwania, P.C.; Shepherd, J.; Wenger, N.K.; Greten, H.; DeMicco, D.A.; Breazna, A.; TNT Investigators. Comparison of 80 versus 10 mg of atorvastatin on occurrence of cardiovascular events after the first event (from the Treating to New Targets [TNT] trial). Am. J. Cardiol. 2010, 105, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Hokanson, J.E.; Austin, M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. J. Cardiovasc. Risk 1996, 3, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Stahel, P.; Xiao, C.; Hegele, R.A.; Lewis, G.F. The Atherogenic Dyslipidemia Complex and Novel Approaches to Cardiovascular Disease Prevention in Diabetes. Can. J. Cardiol. 2018, 34, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Woollard, K.J.; Hoang, A.; Mukhamedova, N.; Stirzaker, R.A.; McCormick, S.P.A.; Remaley, A.T.; Sviridov, D.; Chin-Dusting, J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Anthanont, P.; Diffenderfer, M.R.; Polisecki, E.; Asztalos, B.F. Diagnosis and treatment of high density lipoprotein deficiency. Prog. Cardiovasc. Dis. 2016, 59, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabresi, L.; Gomaraschi, M.; Simonelli, S.; Bernini, F.; Franceschini, G. HDL and atherosclerosis: Insights from inherited HDL disorders. Biochim. Biophys. Acta 2015, 1851, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Paavola, T.; Kuusisto, S.; Jauhiainen, M.; Kakko, S.; Kangas-Kontio, T.; Metso, J.; Soininen, P.; Ala-Korpela, M.; Bloigu, R.; Hannuksela, M.L.; et al. Impaired HDL2-mediated cholesterol efflux is associated with metabolic syndrome in families with early onset coronary heart disease and low HDL-cholesterol level. PLoS ONE 2017, 12, e0171993. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, T.R.; Hu, S.W.; Tian, D.; Li, C.; Zhong, J.K.; Sun, H.G.; Luo, T.T.; Lai, W.Y.; Guo, Z.-G. Acute coronary syndrome remodels the protein cargo and functions of high-density lipoprotein subfractions. PLoS ONE 2014, 9, e94264. [Google Scholar] [CrossRef] [PubMed]
- Tiozzo, E.; Gardener, H.; Hudson, B.I.; Dong, C.; Della-Morte, D.; Crisby, M.; Goldberg, R.B.; Elkind, M.S.V.; Cheung, Y.K.; Wright, C.B.; et al. Subfractions of High-Density Lipoprotein-Cholesterol and Carotid Intima-Media Thickness. Stroke 2016, 47, 1508–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.S.; Khokhar, A.A.; May, H.T.; Kulkarni, K.R.; Blaha, M.J.; Joshi, P.H.; Toth, P.P.; Muhlestein, J.B.; Anderson, J.L.; Knight, S.; et al. Lipoprotein Investigators Collaborative (LIC) HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: The Lipoprotein Investigators Collaborative. Eur. Heart J. 2015, 36, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.H.; Toth, P.P.; Lirette, S.T.; Griswold, M.E.; Massaro, J.M.; Martin, S.S.; Blaha, M.J.; Kulkarni, K.R.; Khokhar, A.A.; Correa, A.; et al. Lipoprotein Investigators Collaborative (LIC) Study Group Association of high-density lipoprotein subclasses and incident coronary heart disease: The Jackson Heart and Framingham Offspring Cohort Studies. Eur. J. Prev. Cardiol. 2016, 23, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Warnick, G.R.; Nauck, M.; Rifai, N. Evolution of methods for measurement of HDL-cholesterol: From ultracentrifugation to homogeneous assays. Clin. Chem. 2001, 47, 1579–1596. [Google Scholar] [PubMed]
- Usui, S.; Nakamura, M.; Jitsukata, K.; Nara, M.; Hosaki, S.; Okazaki, M. Assessment of between-instrument variations in a HPLC method for serum lipoproteins and its traceability to reference methods for total cholesterol and HDL-cholesterol. Clin. Chem. 2000, 46, 63–72. [Google Scholar] [PubMed]
- Jiménez, B.; Holmes, E.; Heude, C.; Tolson, R.F.; Harvey, N.; Lodge, S.L.; Chetwynd, A.J.; Cannet, C.; Fang, F.; Pearce, J.T.M.; et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by 1H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet Lond. Engl. 2014, 384, 618–625. [Google Scholar] [CrossRef]
- Robertson, J.; Peters, M.J.; McInnes, I.B.; Sattar, N. Changes in lipid levels with inflammation and therapy in RA: A maturing paradigm. Nat. Rev. Rheumatol. 2013, 9, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.B.; Riddoch, C.; Kriemler, S.; Hills, A.P.; Hills, A. Physical activity and cardiovascular risk factors in children. Br. J. Sports Med. 2011, 45, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, A.; Hu, P.P. Low High-Density Lipoprotein and Risk of Myocardial Infarction. Clin. Med. Insights Cardiol. 2015, 9, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Superko, H.R.; Pendyala, L.; Williams, P.T.; Momary, K.M.; King, S.B.; Garrett, B.C. High-density lipoprotein subclasses and their relationship to cardiovascular disease. J. Clin. Lipidol. 2012, 6, 496–523. [Google Scholar] [CrossRef] [PubMed]
- Hansel, B.; Giral, P.; Nobecourt, E.; Chantepie, S.; Bruckert, E.; Chapman, M.J.; Kontush, A. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab. 2004, 89, 4963–4971. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; de Faria, E.C.; Chantepie, S.; Chapman, M.J. A normotriglyceridemic, low HDL-cholesterol phenotype is characterised by elevated oxidative stress and HDL particles with attenuated antioxidative activity. Atherosclerosis 2005, 182, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Errico, T.L.; Chen, X.; Martin Campos, J.M.; Julve, J.; Escolà-Gil, J.C.; Blanco-Vaca, F. Basic mechanisms: Structure, function and metabolism of plasma lipoproteins. Clin. Investig. Arterioscler. Publ. Soc. Esp. Arterioscler. 2013, 25, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Fielding, C.J.; Fielding, P.E. Cholesterol transport between cells and body fluids. Role of plasma lipoproteins and the plasma cholesterol esterification system. Med. Clin. N. Am. 1982, 66, 363–373. [Google Scholar] [CrossRef]
- Vladimirov, S.; Gojkovic, T.; Spasojevic-Kalimanovska, V.; Zeljkovic, A.; Vekic, J.; Kalimanovska-Ostric, D.; Jelic-Ivanovic, Z. Influence of LCAT and CETP activity on the reverse cholesterol transport and modification of HDL particles in statin-treated coronary artery disease patients and healthy subjects. Atherosclerosis 2017, 263, e217. [Google Scholar] [CrossRef]
- Norum, K.R. The function of lecithin:cholesterol acyltransferase (LCAT). Scand. J. Clin. Lab. Investig. 2017, 77, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Redondo, S.; Martínez-González, J.; Urraca, C.; Tejerina, T. Emerging therapeutic strategies to enhance HDL function. Lipids Health Dis. 2011, 10, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, G.F. Determinants of plasma HDL concentrations and reverse cholesterol transport. Curr. Opin. Cardiol. 2006, 21, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Cuchel, M.; Lund-Katz, S.; de la Llera-Moya, M.; Millar, J.S.; Chang, D.; Fuki, I.; Rothblat, G.H.; Phillips, M.C.; Rader, D.J. Pathways by which reconstituted high-density lipoprotein mobilizes free cholesterol from whole body and from macrophages. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Julve, J.; Llaverias, G.; Blanco-Vaca, F.; Escolà-Gil, J.C. Seeking novel targets for improving in vivo macrophage-specific reverse cholesterol transport: Translating basic science into new therapies for the prevention and treatment of atherosclerosis. Curr. Vasc. Pharmacol. 2011, 9, 220–237. [Google Scholar] [CrossRef] [PubMed]
- Cordero, A.; Moreno-Arribas, J.; Bertomeu-González, V.; Agudo, P.; Miralles, B.; Masiá, M.D.; López-Palop, R.; Bertomeu-Martínez, V. Low levels of high-density lipoproteins cholesterol are independently associated with acute coronary heart disease in patients hospitalized for chest pain. Rev. Esp. Cardiol. Engl. Ed. 2012, 65, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Qi, Y.; Zhao, D. A meta-analysis on the association between high-density lipoprotein particle subfractions and cardiovascular disease events. Zhonghua Xin Xue Guan Bing Za Zhi 2014, 42, 57–61. [Google Scholar] [PubMed]
- Boekholdt, S.M.; Arsenault, B.J.; Hovingh, G.K.; Mora, S.; Pedersen, T.R.; Larosa, J.C.; Welch, K.M.A.; Amarenco, P.; Demicco, D.A.; Tonkin, A.M.; et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: A meta-analysis. Circulation 2013, 128, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Pandey, A.; Negi, H.; Shafiq, N.; Reddy, S.; Kaur, H.; Chadha, N.; Malhotra, S. Effect of HDL-raising drugs on cardiovascular outcomes: A systematic review and meta-regression. PLoS ONE 2014, 9, e94585. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; Vilahur, G.; Cimmino, G.; Speidl, W.S.; Pinero, A.; Choi, B.G.; Zafar, M.U.; Santos-Gallego, C.G.; Krause, B.; Badimon, L.; et al. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein A-I Milano (ETC-216) administration: Magnetic resonance imaging study in an experimental model of atherosclerosis. J. Am. Coll. Cardiol. 2008, 51, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Barter, P.J.; Rosenson, R.S.; Boden, W.E.; Chapman, M.J.; Cuchel, M.; D’Agostino, R.B.; Davidson, M.H.; Davidson, W.S.; Heinecke, J.W.; et al. High-density lipoproteins: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 484–525. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.-H.; Pedersen, F.K.; Nielsen, C.H.; Müller, K.G. Lipoprotein cholesterol fractions are related to markers of inflammation in children and adolescents with juvenile idiopathic arthritis: A cross sectional study. Pediatr. Rheumatol. Online J. 2016, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namiri-Kalantari, R.; Gao, F.; Chattopadhyay, A.; Wheeler, A.A.; Navab, K.D.; Farias-Eisner, R.; Reddy, S.T. The dual nature of HDL: Anti-Inflammatory and pro-Inflammatory. BioFactors Oxf. Engl. 2015, 41, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Dodani, S.; Kaur, R.; Reddy, S.; Reed, G.L.; Navab, M.; George, V. Can dysfunctional HDL explain high coronary artery disease risk in South Asians? Int. J. Cardiol. 2008, 129, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Ng, C.; Hama, S.; Eliseo, A.J.; Barnard, R.J. Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J. Appl. Physiol. 1985 2006, 101, 1727–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perségol, L.; Vergès, B.; Gambert, P.; Duvillard, L. Inability of HDL from abdominally obese subjects to counteract the inhibitory effect of oxidized LDL on vasorelaxation. J. Lipid Res. 2007, 48, 1396–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.S.; Robson, M.D.; Yu, L.-M.; Shirodaria, C.C.; Cunnington, C.; Kylintireas, I.; Digby, J.E.; Bannister, T.; Handa, A.; Wiesmann, F.; et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: A randomized, placebo-controlled, magnetic resonance imaging study. J. Am. Coll. Cardiol. 2009, 54, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K.; Negami, M.; Takahashi, E. HDL2-cholesterol/HDL3-cholesterol ratio was associated with insulin resistance, high-molecular-weight adiponectin, and components for metabolic syndrome in Japanese. Diabetes Res. Clin. Pract. 2014, 106, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Arts, E.; Fransen, J.; Lemmers, H.; Stalenhoef, A.; Joosten, L.; van Riel, P.; Popa, C.D. High-density lipoprotein cholesterol subfractions HDL2 and HDL3 are reduced in women with rheumatoid arthritis and may augment the cardiovascular risk of women with RA: A cross-sectional study. Arthritis Res. Ther. 2012, 14, R116. [Google Scholar] [CrossRef] [PubMed]
- Brites, F.D.; Bonavita, C.D.; De Geitere, C.; Cloës, M.; Delfly, B.; Yael, M.J.; Fruchart, J.; Wikinski, R.W.; Castro, G.R. Alterations in the main steps of reverse cholesterol transport in male patients with primary hypertriglyceridemia and low HDL-cholesterol levels. Atherosclerosis 2000, 152, 181–192. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Sacks, F.M.; Salvini, S.; Willett, W.C.; Hennekens, C.H. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N. Engl. J. Med. 1991, 325, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Gao, P.; Pennells, L.; Kaptoge, S.; Caslake, M.; Thompson, A.; Butterworth, A.S.; Sarwar, N.; Wormser, D.; Saleheen, D.; et al. Lipid-related markers and cardiovascular disease prediction. JAMA 2012, 307, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Witztum, J.L.; Steinberg, D. The oxidative modification hypothesis of atherosclerosis: Does it hold for humans? Trends Cardiovasc. Med. 2001, 11, 93–102. [Google Scholar] [CrossRef]
- Fateeva, V.V. Pathogenesis of endothelial dysfunction in cerebral atherosclerosis and their correction. Zh Nevrol Psikhiatr Im S S Korsakova 2017, 117, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; Jaffer, F. Imaging the Intersection of Oxidative Stress, Lipids, and Inflammation: Progress Toward Personalized Care of Atherosclerosis. J. Am. Coll. Cardiol. 2018, 71, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.K.; Goh, B.H.; Yap, W.H. Oxidative Stress in Cardiovascular Diseases: Involvement of Nrf2 Antioxidant Redox Signaling in Macrophage Foam Cells Formation. Int. J. Mol. Sci. 2017, 18, 2336. [Google Scholar] [CrossRef] [PubMed]
- Varadharaj, S.; Kelly, O.J.; Khayat, R.N.; Kumar, P.S.; Ahmed, N.; Zweier, J.L. Role of Dietary Antioxidants in the Preservation of Vascular Function and the Modulation of Health and Disease. Front. Cardiovasc. Med. 2017, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, O.B.; Ajiboye, T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2018, 124, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Huyut, Z.; Beydemir, Ş.; Gülçin, İ. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Melnichenko, A.A.; Orekhov, A.N.; Bobryshev, Y.V. Paraoxonase and atherosclerosis-related cardiovascular diseases. Biochimie 2017, 132, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Gaut, J.P.; Heinecke, J.W. Mechanisms for oxidizing low-density lipoprotein. Insights from patterns of oxidation products in the artery wall and from mouse models of atherosclerosis. Trends Cardiovasc. Med. 2001, 11, 103–112. [Google Scholar] [CrossRef]
- Song, X.; Fischer, P.; Chen, X.; Burton, C.; Wang, J. An apoA-I mimetic peptide facilitates off-loading cholesterol from HDL to liver cells through scavenger receptor BI. Int. J. Biol. Sci. 2009, 5, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Smith, J.D. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat. Genet. 1996, 14, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kelso, G.J.; Stuart, W.D.; Richter, R.J.; Furlong, C.E.; Jordan-Starck, T.C.; Harmony, J.A. Apolipoprotein J is associated with paraoxonase in human plasma. Biochemistry 1994, 33, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Volkova, N.; Aviram, M. HDL3 stimulates paraoxonase 1 antiatherogenic catalytic and biological activities in a macrophage model system: In vivo and in vitro studies. BioFactors Oxf. Engl. 2014, 40, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Stafforini, D.M. Plasma PAF-AH (PLA2G7). Enzymes 2015, 38, 71–93. [Google Scholar] [PubMed]
- Samad, F.; Ruf, W. Inflammation, obesity, and thrombosis. Blood 2013, 122, 3415–3422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, J.; Wu, X.; Luo, Q.; Wei, G.; Xu, M.; Wu, Y.; Liu, Y.; Li, X.; Zi, J.; Ju, W.; et al. NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica 2018. [Google Scholar] [CrossRef] [PubMed]
- Annema, W.; von Eckardstein, A.; Kovanen, P.T. HDL and Atherothrombotic Vascular Disease; Springer: Cham, Switzerland, 2015; pp. 369–403. [Google Scholar]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Castelli, W.P.; Anderson, K.; Wilson, P.W.F.; Levy, D. Lipids and risk of coronary heart disease The Framingham Study. Ann. Epidemiol. 1992, 2, 23–28. [Google Scholar] [CrossRef]
- Sammalkorpi, K.; Valtonen, V.; Kerttula, Y.; Nikkilä, E.; Taskinen, M.R. Changes in serum lipoprotein pattern induced by acute infections. Metabolism 1988, 37, 859–865. [Google Scholar] [CrossRef]
- Viswambharan, H.; Ming, X.-F.; Zhu, S.; Hubsch, A.; Lerch, P.; Vergères, G.; Rusconi, S.; Yang, Z. Reconstituted High-Density Lipoprotein Inhibits Thrombin-Induced Endothelial Tissue Factor Expression Through Inhibition of RhoA and Stimulation of Phosphatidylinositol 3-Kinase but not Akt/Endothelial Nitric Oxide Synthase. Circ. Res. 2004, 94, 918–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, J.H.; Kojima, K.; Banka, C.L.; Curtiss, L.K.; Fernández, J.A. High-density lipoprotein enhancement of anticoagulant activities of plasma protein S and activated protein C. J. Clin. Investig. 1999, 103, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holy, E.; Besler, C.; Reiner, M.; Camici, G.; Manz, J.; Beer, J.; Lüscher, T.; Landmesser, U.; Tanner, F. High-density lipoprotein from patients with coronary heart disease loses anti-thrombotic effects on endothelial cells: Impact on arterial thrombus formation. Thromb. Haemost. 2014, 112, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Calkin, A.C.; Drew, B.G.; Ono, A.; Duffy, S.J.; Gordon, M.V.; Schoenwaelder, S.M.; Sviridov, D.; Cooper, M.E.; Kingwell, B.A.; Jackson, S.P. Reconstituted High-Density Lipoprotein Attenuates Platelet Function in Individuals with Type 2 Diabetes Mellitus by Promoting Cholesterol Efflux. Circulation 2009, 120, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Badrnya, S.; Assinger, A.; Volf, I. Native High Density Lipoproteins (HDL) Interfere with Platelet Activation Induced by Oxidized Low Density Lipoproteins (OxLDL). Int. J. Mol. Sci. 2013, 14, 10107–10121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, A.J.; Bijl, N.; Yvan-Charvet, L.; Welch, C.B.; Bhagwat, N.; Reheman, A.; Wang, Y.; Shaw, J.A.; Levine, R.L.; Ni, H.; et al. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat. Med. 2013, 19, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J.; et al. Major Lipids, Apolipoproteins, and Risk of Vascular Disease. JAMA 2009, 302, 1993. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.; Gotto, A.M.; LaRosa, J.C.; Maroni, J.; Szarek, M.; Grundy, S.M.; Kastelein, J.J.P.; Bittner, V.; Fruchart, J.-C. Treating to New Targets Investigators. HDL Cholesterol, Very Low Levels of LDL Cholesterol, and Cardiovascular Events. N. Engl. J. Med. 2007, 357, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Federici, A.B. HDL/ApoA-I: Role in VWF-dependent thrombosis. Blood 2016, 127, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Schaefer, E.J. High-density lipoprotein subpopulations in pathologic conditions. Am. J. Cardiol. 2003, 91, 12E–17E. [Google Scholar] [CrossRef]
- Rodríguez-Carrio, J.; Alperi-López, M.; López, P.; López-Mejías, R.; Alonso-Castro, S.; Abal, F.; Ballina-García, F.J.; González-Gay, M.Á.; Suárez, A. High triglycerides and low high-density lipoprotein cholesterol lipid profile in rheumatoid arthritis: A potential link among inflammation, oxidative status, and dysfunctional high-density lipoprotein. J. Clin. Lipidol. 2017, 11, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Ramírez, G.; Pérez-Méndez, O.; López-Osorio, C.; Kuri-Alfaro, J.; Espinola-Zavaleta, N. Effect of the Treatment with Allopurinol on the Endothelial Function in Patients with Hyperuricemia. Endocr. Res. 2012, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramírez, M.; Flores-Castillo, C.; Sánchez-Lozada, L.G.; Bautista-Pérez, R.; Carreón-Torres, E.; Fragoso, J.M.; Rodriguez-Pérez, J.M.; García-Arroyo, F.E.; López-Olmos, V.; Luna-Luna, M.; et al. Hyperuricemia is Associated with Increased Apo AI Fractional Catabolic Rates and Dysfunctional HDL in New Zealand Rabbits. Lipids 2017, 52, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A.K. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol. Cell. Biochem. 2018, 440, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Carnuta, M.G.; Stancu, C.S.; Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Popescu, A.C.; Popescu, M.R.; Vlad, A.; Dimulescu, D.R.; et al. Dysfunctional high-density lipoproteins have distinct composition, diminished anti-inflammatory potential and discriminate acute coronary syndrome from stable coronary artery disease patients. Sci. Rep. 2017, 7, 7295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Méndez, Ó.; Pacheco, H.G.; Martínez-Sánchez, C.; Franco, M. HDL-cholesterol in coronary artery disease risk: Function or structure? Clin. Chim. Acta 2014, 429, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Vergeer, M.; Holleboom, A.G.; Kastelein, J.J.P.; Kuivenhoven, J.A. The HDL hypothesis: Does high-density lipoprotein protect from atherosclerosis? J. Lipid Res. 2010, 51, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Tang, C.; Guevara, M.E.; Wei, H.; Wietecha, T.; Shao, B.; Subramanian, S.; Omer, M.; Wang, S.; O’Brien, K.D.; et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Investig. 2016, 126, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Schwertani, A.; Choi, H.Y.; Genest, J. HDLs and the pathogenesis of atherosclerosis. Curr. Opin. Cardiol. 2018, 33, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Annema, W.; von Eckardstein, A. Dysfunctional high-density lipoproteins in coronary heart disease: Implications for diagnostics and therapy. Transl. Res. 2016, 173, 30–57. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Cha, K.S.; Lee, H.W.; Oh, J.-H.; Choi, J.H.; Lee, H.C.; Hong, T.J.; Jeong, M.H.; Chae, S.C.; Kim, Y.J. Predictive and protective role of high-density lipoprotein cholesterol in acute myocardial infarction. Cardiol. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Talbot, D.; Delaney, J.A.C.; Sandfort, V.; Herrington, D.M.; McClelland, R.L. Importance of the lipid-related pathways in the association between statins, mortality, and cardiovascular disease risk: The Multi-Ethnic Study of Atherosclerosis. Pharmacoepidemiol. Drug Saf. 2018, 27, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Karlson, B.W.; Palmer, M.K.; Nicholls, S.J.; Barter, P.J.; Lundman, P. Effects of age, gender and statin dose on lipid levels: Results from the VOYAGER meta-analysis database. Atherosclerosis 2017, 265, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Postmus, I.; Warren, H.R.; Trompet, S.; Arsenault, B.J.; Avery, C.L.; Bis, J.C.; Chasman, D.I.; de Keyser, C.E.; Deshmukh, H.A.; Evans, D.S.; et al. Meta-analysis of genome-wide association studies of HDL cholesterol response to statins. J. Med. Genet. 2016, 53, 835–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adiels, M.; Chapman, M.J.; Robillard, P.; Krempf, M.; Laville, M.; Borén, J. Niacin Study Group Niacin action in the atherogenic mixed dyslipidemia of metabolic syndrome: Insights from metabolic biomarker profiling and network analysis. J. Clin. Lipidol. 2018, 12, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Jones, S.R.; Slee, A.; Fleg, J.; Marcovina, S.M.; Lacy, M.; McBride, R.; Boden, W.E. Relationship between lipoprotein subfraction cholesterol and residual risk for cardiovascular outcomes: A post hoc analysis of the AIM-HIGH trial. J. Clin. Lipidol. 2018, 12, 741–747.e11. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, B.; Tao, W.; Hao, Z.; Liu, M. Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst. Rev. 2015, CD009580. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Saver, J.L.; Towfighi, A.; Chow, J.; Ovbiagele, B. Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: A meta-analysis. Atherosclerosis 2011, 217, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Farnier, M.; Guyton, J.R.; Jensen, E.; Polis, A.B.; Johnson-Levonas, A.O.; Brudi, P. Effects of ezetimibe, simvastatin and ezetimibe/simvastatin on correlations between apolipoprotein B, LDL cholesterol and non-HDL cholesterol in patients with primary hypercholesterolemia. Atherosclerosis 2013, 229, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.; Zhou, F.; Chen, C.; Zhou, L.; Li, Y.; Liu, L.; Pei, F.; Luo, H.; Hu, Z.; et al. Cholesteryl ester transfer protein inhibitors in the treatment of dyslipidemia: A systematic review and meta-analysis. PLoS ONE 2013, 8, e77049. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Kei, A.; Elisaf, M.S. Anacetrapib, a New CETP Inhibitor: The New Tool for the Management of Dyslipidemias? Diseases 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Collins, D.; Horvath, K.V.; Bloomfield, H.E.; Robins, S.J.; Schaefer, E.J. Relation of gemfibrozil treatment and high-density lipoprotein subpopulation profile with cardiovascular events in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Metabolism 2008, 57, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F. NLA Symposium on High Density Lipoproteins High-density lipoprotein particles, coronary heart disease, and niacin. J. Clin. Lipidol. 2010, 4, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.G.; Zhao, X.-Q.; Chait, A.; Fisher, L.D.; Cheung, M.C.; Morse, J.S.; Dowdy, A.A.; Marino, E.K.; Bolson, E.L.; Alaupovic, P.; et al. Simvastatin and Niacin, Antioxidant Vitamins, or the Combination for the Prevention of Coronary Disease. N. Engl. J. Med. 2001, 345, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Villines, T.C.; Stanek, E.J.; Devine, P.J.; Griffen, L.; Miller, M.; Weissman, N.J.; Turco, M. Extended-Release Niacin or Ezetimibe and Carotid Intima–Media Thickness. N. Engl. J. Med. 2009, 361, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Julius, U.; Fischer, S. Nicotinic acid as a lipid-modifying drug—A review. Atheroscler. Suppl. 2013, 14, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bala, I.M.; Lifshits, V.M.; Sidel’nikova, V.I. Granulocytic chalone and antichalone as homeostatic factors in the functional system of the blood in aseptic inflammation. Patol. Fiziol. Eksp. Ter. 1988, 39–41. [Google Scholar]
- Carballo-Jane, E.; Chen, Z.; O’Neill, E.; Wang, J.; Burton, C.; Chang, C.H.; Chen, X.; Eveland, S.; Frantz-Wattley, B.; Gagen, K.; et al. ApoA-I mimetic peptides promote pre-β HDL formation in vivo causing remodeling of HDL and triglyceride accumulation at higher dose. Bioorg. Med. Chem. 2010, 18, 8669–8678. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J. Liver X Receptors (LXR) as Therapeutic Targets in Dyslipidemia. Cardiovasc. Ther. 2008, 26, 297–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mencarelli, A.; Fiorucci, S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J. Cell. Mol. Med. 2010, 14, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Moore, K.J. MicroRNA Control of High-Density Lipoprotein Metabolism and Function. Circ. Res. 2014, 114, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Hama, S.; Hough, G.; Grijalva, V.R.; Yu, N.; Ansell, B.J.; Datta, G.; Garber, D.W.; et al. Apolipoprotein A-I Mimetic Peptides. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1325–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remaley, A.T.; Amar, M.; Sviridov, D. HDL-replacement therapy: Mechanism of action, types of agents and potential clinical indications. Expert Rev. Cardiovasc. Ther. 2008, 6, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, L.; de Vries-van der Weij, J.; Zadelaar, S.; Kleemann, R.; Kooistra, T. LXR agonist suppresses atherosclerotic lesion growth and promotes lesion regression in apoE*3Leiden mice: Time course and mechanisms. J. Lipid Res. 2009, 50, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Hafiane, A.; Genest, J. HDL, Atherosclerosis, and Emerging Therapies. Cholesterol 2013, 2013, 891403. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Ediriweera, H.; Afonso, M.S.; Ramkhelawon, B.; Singaravelu, R.; Liao, X.; Bandler, R.C.; Rahman, K.; Fisher, E.A.; Rayner, K.J.; et al. microRNA-33 Regulates Macrophage Autophagy in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Van de Woestijne, A.P.; van der Graaf, Y.; Liem, A.-H.; Cramer, M.J.M.; Westerink, J.; Visseren, F.L.J.; SMART Study Group. Low High-Density Lipoprotein Cholesterol Is Not a Risk Factor for Recurrent Vascular Events in Patients with Vascular Disease on Intensive Lipid-Lowering Medication. J. Am. Coll. Cardiol. 2013, 62, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Tibolla, G.; Norata, G.D.; Catapano, A.L. HDL: To Treat or Not To Treat? Curr. Atheroscler. Rep. 2014, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Luna, D.; Martínez-Hinojosa, E.; Cancino-Diaz, J.C.; Belefant-Miller, H.; López-Rodríguez, G.; Betanzos-Cabrera, G. Daily supplementation with fresh pomegranate juice increases paraoxonase 1 expression and activity in mice fed a high-fat diet. Eur. J. Nutr. 2018, 57, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Stowe, C.B. The effects of pomegranate juice consumption on blood pressure and cardiovascular health. Complement. Ther. Clin. Pract. 2011, 17, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Penugonda, K. Pomegranate juice: A heart-healthy fruit juice. Nutr. Rev. 2009, 67, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic. Biol. Med. 2004, 37, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Arrol, S.; Durrington, P.N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991, 286, 152–154. [Google Scholar] [CrossRef] [Green Version]
- Oda, M.N.; Bielicki, J.K.; Ho, T.T.; Berger, T.; Rubin, E.M.; Forte, T.M. Paraoxonase 1 Overexpression in Mice and Its Effect on High-Density Lipoproteins. Biochem. Biophys. Res. Commun. 2002, 290, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Ikhlef, S.; Berrougui, H.; Kamtchueng Simo, O.; Zerif, E.; Khalil, A. Human paraoxonase 1 overexpression in mice stimulates HDL cholesterol efflux and reverse cholesterol transport. PLoS ONE 2017, 12, e0173385. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Shukla, R.; Venkata Madhu, S.; Kaur Gambhir, J.; Madhava Prabhu, K. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin. Biochem. 2003, 36, 557–562. [Google Scholar] [CrossRef]
- Rosenblat, M.; Hayek, T.; Aviram, M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis 2006, 187, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E–deficient mice. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Paraoxonase 1 and atherosclerosis: Is the gene or the protein more important? Free Radic. Biol. Med. 2004, 37, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Leckey, L.C.; Garige, M.; Varatharajalu, R.; Gong, M.; Nagata, T.; Spurney, C.F.; Lakshman, R.M. Quercetin and Ethanol Attenuate the Progression of Atherosclerotic Plaques with Concomitant Up Regulation of Paraoxonase1 (PON1) Gene Expression and PON1 Activity in LDLR−/− Mice. Alcohol. Clin. Exp. Res. 2010, 34, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.D.; Bansal, M.P. Studies on HDL associated enzymes under experimental hypercholesterolemia: Possible modulation on selenium supplementation. Lipids Health Dis. 2009, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Koukos, G.; Chroni, A.; Duka, A.; Kardassis, D.; Zannis, V.I. Naturally occurring and bioengineered apoA-I mutations that inhibit the conversion of discoidal to spherical HDL: The abnormal HDL phenotypes can be corrected by treatment with LCAT. Biochem. J. 2007, 406, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Fawole, O.A.; Opara, U.L. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate co-products subjected to in vitro digestion. BMC Complement. Altern. Med. 2016, 16, 358. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W. Health effects of vegetables and fruit: Assessing mechanisms of action in human experimental studies. Am. J. Clin. Nutr. 1999, 70, 475s–490s. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Volkova, N.; Coleman, R.; Dreher, M.; Reddy, M.K.; Ferreira, D.; Rosenblat, M. Pomegranate Phenolics from the Peels, Arils, and Flowers Are Antiatherogenic: Studies in Vivo in Atherosclerotic Apolipoprotein E-Deficient (E0) Mice and in Vitro in Cultured Macrophages and Lipoproteins. J. Agric. Food Chem. 2008, 56, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Padró, T.; Casaní, L.; Mendieta, G.; López, J.A.; Streitenberger, S.; Badimon, L. Polyphenol-enriched Diet Prevents Coronary Endothelial Dysfunction by Activating the Akt/eNOS Pathway. Rev. Esp. Cardiol. Engl. Ed. 2015, 68, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Martínez-González, J.; Llorente-Cortés, V.; Rodríguez, C.; Padró, T. Cell biology and lipoproteins in atherosclerosis. Curr. Mol. Med. 2006, 6, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Sattar, N. Triglycerides and coronary heart disease: Have recent insights yielded conclusive answers? Curr. Opin. Lipidol. 2009, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, A.G.; Dige, A.; Krog, J.; Tønnesen, E.; Wogensen, L. Soluble adhesion molecules correlate with surface expression in an in vitro model of endothelial activation. Basic Clin. Pharmacol. Toxicol. 2013, 113, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Perségol, L.; Darabi, M.; Dauteuille, C.; Lhomme, M.; Chantepie, S.; Rye, K.-A.; Therond, P.; Chapman, M.J.; Salvayre, R.; Nègre-Salvayre, A.; et al. Small dense HDLs display potent vasorelaxing activity, reflecting their elevated content of sphingosine-1-phosphate. J. Lipid Res. 2018, 59, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zheng, J.-B.; Ai, W.-T.; Yao, X.-W.; Liang, L.; Cheng, G.; Shou, X.-L.; Sun, C.-F. Felodipine inhibits ox-LDL-induced reactive oxygen species production and inflammation in human umbilical vein endothelial cells. Mol. Med. Rep. 2017, 16, 4871–4878. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Larson, M.G.; Beiser, A.; Levy, D. Lifetime risk of developing coronary heart disease. Lancet 1999, 353, 89–92. [Google Scholar] [CrossRef]
- Stalenhoef, A.F.; de Graaf, J. Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL. Curr. Opin. Lipidol. 2008, 19, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Jagla, A.; Schrezenmeir, J. Postprandial triglycerides and endothelial function. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2001, 109, S533–S547. [Google Scholar] [CrossRef]
- Van Eck, M.; Bos, I.S.T.; Kaminski, W.E.; Orso, E.; Rothe, G.; Twisk, J.; Bottcher, A.; Van Amersfoort, E.S.; Christiansen-Weber, T.A.; Fung-Leung, W.-P.; et al. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 6298–6303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuai, R.; Li, D.; Chen, Y.E.; Moon, J.J.; Schwendeman, A. High-Density Lipoproteins: Nature’s Multifunctional Nanoparticles. ACS Nano 2016, 10, 3015–3041. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-Y.; Jiang, J.-G.; Huang, C.-L.; Zhu, W.; Zheng, C.-Y. Polyphenols from Blossoms of Citrus aurantium L. var. amara Engl. Show Significant Anti-Complement and Anti-Inflammatory Effects. J. Agric. Food Chem. 2017, 65, 9061–9068. [Google Scholar] [CrossRef] [PubMed]
- Danesi, F.; Ferguson, L. Could Pomegranate Juice Help in the Control of Inflammatory Diseases? Nutrients 2017, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L. Anti-Inflammatory Agents and Antioxidants as a Possible “Third Great Wave” in Cardiovascular Secondary Prevention. Am. J. Cardiol. 2008, 101, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tousoulis, D.; Tsigkou, V.; Kokkou, E.; Oikonomou, E.; Vavuranakis, M.; Basdra, E.K.; Papavassiliou, A.G.; Stefanadis, C. Flavonoids in atherosclerosis: An overview of their mechanisms of action. Curr. Med. Chem. 2013, 20, 2641–2660. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Amouyel, P.; Dallongeville, J. Fruits, vegetables and coronary heart disease. Nat. Rev. Cardiol. 2009, 6, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Arós, F.; Estruch, R. Mediterranean Diet and Cardiovascular Prevention. Rev. Esp. Cardiol. Engl. Ed. 2013, 66, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Delgado, N.T.B.; Rouver, W.N.; Freitas-Lima, L.C.; de Paula, T.D.-C.; Duarte, A.; Silva, J.F.; Lemos, V.S.; Santos, A.M.C.; Mauad, H.; Santos, R.L.; et al. Pomegranate Extract Enhances Endothelium-Dependent Coronary Relaxation in Isolated Perfused Hearts from Spontaneously Hypertensive Ovariectomized Rats. Front. Pharmacol. 2016, 7, 522. [Google Scholar] [CrossRef] [PubMed]
- Olivero-David, R.; Ruiz-Roso, M.B.; Caporaso, N.; Perez-Olleros, L.; De Las Heras, N.; Lahera, V.; Ruiz-Roso, B. In vivo bioavailability of polyphenols from grape by-product extracts, and effect on lipemia of normocholesterolemic Wistar rats. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nauman, M.; Kale, R.K.; Singh, R.P. Polyphenols of Salix aegyptiaca modulate the activities of drug metabolizing and antioxidant enzymes, and level of lipid peroxidation. BMC Complement. Altern. Med. 2018, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green Tea Polyphenols Modulate Colonic Microbiota Diversity and Lipid Metabolism in High-Fat Diet Treated HFA Mice. J. Food Sci. 2018, 83, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Kuvin, J.T.; Dave, D.M.; Sliney, K.A.; Mooney, P.; Patel, A.R.; Kimmelstiel, C.D.; Karas, R.H. Effects of extended-release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease. Am. J. Cardiol. 2006, 98, 743–745. [Google Scholar] [CrossRef] [PubMed]
- van der Hoorn, J.W.A.; de Haan, W.; Berbee, J.F.P.; Havekes, L.M.; Jukema, J.W.; Rensen, P.C.N.; Princen, H.M.G. Niacin Increases HDL by Reducing Hepatic Expression and Plasma Levels of Cholesteryl Ester Transfer Protein in APOE*3Leiden.CETP Mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2016–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilahur, G.; Badimon, L. Antiplatelet properties of natural products. Vasc. Pharmacol. 2013, 59, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Deckelbaum, R.J. Omega-3 fatty acids: Mechanisms underlying “protective effects” in atherosclerosis. Curr. Opin. Lipidol. 2013, 24, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Catalán, U.; Fernández-Castillejo, S.; Pons, L.; Heras, M.; Aragonés, G.; Anglès, N.; Morelló, J.-R.; Solà, R. Alpha-tocopherol and BAY 11-7082 reduce vascular cell adhesion molecule in human aortic endothelial cells. J. Vasc. Res. 2012, 49, 319–328. [Google Scholar] [CrossRef] [PubMed]
- van Dam, B.; van Hinsbergh, V.W.M.; Stehouwer, C.D.A.; Versteilen, A.; Dekker, H.; Buytenhek, R.; Princen, H.M.; Schalkwijk, C.G. Vitamin E inhibits lipid peroxidation-induced adhesion molecule expression in endothelial cells and decreases soluble cell adhesion molecules in healthy subjects. Cardiovasc. Res. 2003, 57, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Palozza, P.; Parrone, N.; Simone, R.E.; Catalano, A. Lycopene in atherosclerosis prevention: An integrated scheme of the potential mechanisms of action from cell culture studies. Arch. Biochem. Biophys. 2010, 504, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.A.; Parikh, M.; Patel, K.V.; Patel, K.G.; Joshi, C.G.; Gandhi, T.R. Evaluation of the effect of Punica granatum juice and punicalagin on NFκB modulation in inflammatory bowel disease. Mol. Cell. Biochem. 2016, 419, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Dornfeld, L.; Kaplan, M.; Coleman, R.; Gaitini, D.; Nitecki, S.; Hofman, A.; Rosenblat, M.; Volkova, N.; Presser, D.; et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: Studies in atherosclerotic mice and in humans. Drugs Exp. Clin. Res. 2002, 28, 49–62. [Google Scholar] [PubMed]



| Drug | Effect of Drugs on HDL-C and Cardiovascular Health | Author |
|---|---|---|
| Statins | Women and men (32,258) obtained from the (an individual patient data meta-analysis of statin therapY in at risk Groups: | |
| Effects of rosuvastatin, atorvastatin and simvastatin) VOYAGER study who received atorvastatin (10–80 mg), rosuvastatin (5–40 mg) or simvastatin (10–80 mg); all statins and doses decreased the concentration of LDL-C and increased the HDL-C. | [102] | |
| A meta-analysis of genome-wide association in a population of European descendents was made to identify variants that modify HDL-C. Participants (27,720) showed an association between the cholesterol ester transporter protein CETP locus (chromosome 16) and HDL-C response to statin treatment. | [103] | |
| Nicotinic acid | Obese, nondiabetic, hypertriglyceridemic males (19) with low HDL-C levels received nicotinic acid for eight weeks, achieving a decrease in biomarkers of inflammation, cell adhesion and cell proliferation in addition to LDL-C and total cholesterol. | [104] |
| AIM-HIGH individuals (2457) with cardiovascular disease at baseline and one year of treatment of extended-release niacin and high triglycerides (>200 mg/dL) and very low HDL-C (<32 mg/dL) showed a significant reduction in serum levels of remnant lipoprotein cholesterol and increased HDL2-C. | [105] | |
| Fibrates | Participants of a Cochrane Collaboration study (16,112) showed a protective effect of fibrates and safety in the secondary prevention of different cardiovascular events including coronary and cerebrovascular disease. | [106] |
| In individuals with hypertriglyceridemia (7389) and 5068 individuals with hypertriglyceridemia and low HDL-C levels, the treatment with fibrates reduced the subsequent vascular event risk. | [107] | |
| Bile acid binding resins | Patients treated with ezetimibe (10 mg/day) (302), 1234 patients treated with simvastatin (10 mg/day, 20 mg/day, 40 mg/day or 80 mg/day) and 1236 patients with combination of both drugs (10/10 mg/day, 10/20 mg/day, 10/40 mg/day or 10/80 mg/day), are associated with smaller decreases in Apo B compared with LDL-C and non-HDL-C. | [108] |
| CETP inhibitors | Patients with increased HDL-C (2826), and 3739 patients with reduced triglyceride levels and LDL-C showed an increase in blood pressure due to the increase of LDL metabolism through their receptors. Different CETP inhibitors (mainly anacetrapib) are used to increase HDL-C and Apo-A1 levels and significantly alter HDL2 subclasses and pre-β HDL particles with a decrease in LDL-C. | [109,110] |
| Proteins Interacting with HDL3 | Effects on HDL3 | Reference |
|---|---|---|
| PON1 | PON1 increases macrophage cholesterol efflux and improves the antioxidant properties of HDL. | [71] |
| PAF-AH | PAF-AH activity and expression are upregulated by mediators of inflammation at the transcriptional level; their stability provides antioxidant properties and anti-atherogenic activities to HDL3. | [72,141] |
| LCAT | Supplementation of the enzyme LCAT is a potential therapeutic intervention for HDL abnormalities that result from specific mutations on Apo-A1. | [142] |
| Nutrients | Natural Sources | Mechanism |
|---|---|---|
| Vitamin B3 | Chicken, fish, peanuts, and legumes | Involved in catabolism and synthesis of HDL; increase the production of Apo-A1 and expression of ABCA1; helps to transfer the cholesterol from macrophages to nascent HDL; decreases the expression and activity of CETP [169,170]. |
| Omega 3 fatty acids | Salmon, peas, tuna, sardines, and trout | Reduces the uptake and binding of LDL to the arterial wall due to a reduction of lipoprotein-lipase levels and macrophages; facilitates the incorporation of omega-3 into the phospholipid membrane; changes arachidonic acid metabolism reducing the release of thromboxane A2 [171,172]. |
| Vitamin E (α-, β-, γ-, δ-tocopherol and tocotrienol) | Wheat germ oil, sunflower seeds, almonds, peanuts, corn oil, olive oil, spinach, broccoli, soybean oil, kiwi, mango and tomato | Reduces the expression of VCAM-1, ICAM-1 and e-selectin; decreases the adhesion of leukocytes into the endothelium or arterial wall [173,174]. |
| Lycopene | Tomatoes, grapefruit, watermelon, and papaya | Reduces intima wall thickness or lesions in aorta mainly to its antioxidant activity related to LDL oxidation; inhibits the activity and expression of 3-hydroxy-methyl glutaryl (HMG)-CoA, reducing cholesterol synthesis [175]. |
| Gallic acid, punicalagin | Pomegranate juice | Reduces the expression of inflammatory cytokines TNF-α, IL-1β, IL-18 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κβ) [176]; inhibits LDL oxidation and macrophage foam cell formation due to the accumulation of these polyphenols in arterial macrophages besides inactive ROS and reactive nitrogen species (RNS) [177]. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Luna, D.; Ortiz-Rodriguez, M.A.; Medina-Briseño, L.; Carreón-Torres, E.; Izquierdo-Vega, J.A.; Sharma, A.; Cancino-Díaz, J.C.; Pérez-Méndez, O.; Belefant-Miller, H.; Betanzos-Cabrera, G. Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease. Molecules 2018, 23, 2730. https://doi.org/10.3390/molecules23112730
Estrada-Luna D, Ortiz-Rodriguez MA, Medina-Briseño L, Carreón-Torres E, Izquierdo-Vega JA, Sharma A, Cancino-Díaz JC, Pérez-Méndez O, Belefant-Miller H, Betanzos-Cabrera G. Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease. Molecules. 2018; 23(11):2730. https://doi.org/10.3390/molecules23112730
Chicago/Turabian StyleEstrada-Luna, Diego, María Araceli Ortiz-Rodriguez, Lizett Medina-Briseño, Elizabeth Carreón-Torres, Jeannett Alejandra Izquierdo-Vega, Ashutosh Sharma, Juan Carlos Cancino-Díaz, Oscar Pérez-Méndez, Helen Belefant-Miller, and Gabriel Betanzos-Cabrera. 2018. "Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease" Molecules 23, no. 11: 2730. https://doi.org/10.3390/molecules23112730
APA StyleEstrada-Luna, D., Ortiz-Rodriguez, M. A., Medina-Briseño, L., Carreón-Torres, E., Izquierdo-Vega, J. A., Sharma, A., Cancino-Díaz, J. C., Pérez-Méndez, O., Belefant-Miller, H., & Betanzos-Cabrera, G. (2018). Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease. Molecules, 23(11), 2730. https://doi.org/10.3390/molecules23112730