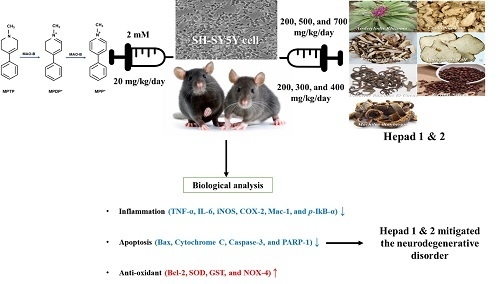
Mitigation Effects of a Novel Herbal Medicine, Hepad, on Neuroinflammation, Neuroapoptosis, and Neuro-Oxidation
Abstract
:1. Introduction
2. Results
2.1. Hepad Ameliorates the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine Hydrochloride (MPTP)-Induced Reduction in Neuronal Cell Viability
2.2. Hepad Attenuates MPTP-Induced Inflammation
2.3. Effects of Hepad on Pro-Apoptotic and Anti-Apoptotic Protein Expression in MPTP-Intoxicated SH-SY5Y Cells
2.4. Hepad Suppresses MPTP-Induced Oxidative Stress
2.5. Hepad Attenuates the Elevation of Phosphorylated Protein Kinase B (p-AKT) and Mitogen-Activated Protein Kinase (MAPK) in MPTP-Intoxicated SH-SY5Y Cells
2.6. Hematoxylin and Eosin (H&E) Staining and Immunohistochemical Detection of Tyrosine Hydroxylase (TH) in the substantia nigra (SN) of Mice
2.7. Mitigation Effects of Hepad on MPTP-Induced Inflammatory Responses
2.8. Hepad Affects the Activation of Pro-Apoptotic and Anti-Apoptotic Proteins
2.9. Hepad Inhibits MPTP-Induced Oxidative Stress
2.10. Hepad Modulates the p-AKT and MAPK Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Cell Culture
4.3. Cell Viability
4.4. Animal Experiments
4.5. Histological Analysis
4.6. Immunohistochemical Staining
4.7. Western Blotting Analysis
4.8. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lev, N.; Melamed, E.; Offen, D. Apoptosis and Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 245–250. [Google Scholar] [CrossRef]
- Grimmig, B.; Daly, L.; Subbarayan, M.; Hudson, C.; Williamson, R.; Nash, K.; Bickford, P.C. Astaxanthin is neuroprotective in an aged mouse model of Parkinson’s disease. Oncotarget. 2018, 9, 10388–10401. [Google Scholar] [CrossRef] [PubMed]
- Fiskum, G.; Starkov, A.; Polster, B.M.; Chinopoulos, C. Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson’s disease. Ann. N. Y. Acad. Sci 2003, 991, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Ko, H.M.; Koppula, S.; Kim, B.W.; Choi, D.K. Protective effect of Chrysanthemum indicum Linne against 1-methyl-4-phenylpridinium ion and lipopolysaccharide-induced cytotoxicity in cellular model of Parkinson’s disease. Food Chem. Toxicol. 2011, 49, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, O.; Schulz, J.B. Apoptotic mechanisms and antiapoptotic therapy in the MPTP model of Parkinson’s disease. Toxicol. Lett. 2003, 139, 135–151. [Google Scholar] [CrossRef]
- Przedborski, S.; Jackson-Lewis, V. Mechanisms of MPTP toxicity. Mov. Disord. 1998, 13, 35–38. [Google Scholar] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Offen, D.; Elkon, H.; Melamed, E. Apoptosis as a general cell death pathway in neurodegenerative diseases. J. Neural. Transm. Suppl. 2000, 58, 153–166. [Google Scholar]
- Liu, B.; Hong, J.S. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef] [PubMed]
- González-Scarano, F.; Baltuch, G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci 1999, 22, 219–240. [Google Scholar] [CrossRef] [PubMed]
- McGeer, E.G.; McGeer, P.L. The role of anti-inflammatory agents in Parkinson’s disease. CNS Drugs 2007, 21, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Nishioku, T.; Matsumoto, J.; Dohgu, S.; Sumi, N.; Miyao, K.; Takata, F.; Shuto, H.; Yamauchi, A.; Kataoka, Y. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J. Pharmacol. Sci. 2010, 112, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Kitamura, Y. Molecular approaches to the treatment, prophylaxis, and diagnosis of Alzheimer’s disease: Tangle formation, amyloid-beta, and microglia in Alzheimer’s disease. J. Pharmacol. Sci. 2012, 118, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.; Zhang, L.; Chen, Y.; Zhang, Y.; Zhang, F.; Zhou, X.; Zhang, X.; Gao, B.; Zhen, X.; Zhang, J.; et al. Anti-inflammatory effects of glaucocalyxin B in microglia cells. J. Pharmacol. Sci. 2015, 128, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Jeohn, G.H.; Cooper, C.L.; Wilson, B.; Chang, R.C.; Jang, K.J.; Kim, H.C.; Liu, B.; Hong, J.S. p38 MAP kinase is involved in lipopolysaccharide-induced dopaminergic neuronal cell death in rat mesencephalic neuron-glia cultures. Ann. N. Y. Acad. Sci. 2002, 962, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.Y.; Lee, N.R.; Kim, D.H.; Gu, A.; Kim, S.Y.; Song, D.Y.; Kim, D.H.; Choi, H.J.; Park, B.J.; Kim, I.S. Protective effect of a novel herbmedicine, Hepad, on apoptosis of SH-SY5Y cells and a rat model of Parkinson’s disease. Mol. Cell. Toxicol. 2015, 11, 223–230. [Google Scholar] [CrossRef]
- Kim, M.I.; Kim, J.H.; Syed, A.S.; Kim, Y.M.; Choe, K.K.; Kim, C.Y. Application of centrifugal partition chromatography for bioactivity-guided purification of antioxidant-response-element-inducing constituents from Atractylodis Rhizoma Alba. Molecules 2018, 23, 2274. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.S.; Ha, B.G.; Shon, Y.H. Effect of Cnidii Rhizoma on nitric oxide production and invasion of human colorectal adenocarcinoma HT-29 cells. Oncol. Lett. 2015, 9, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, C.S.; Lim, Y.; Kim, H.S. Paeonia japonica, Houttuynia cordata, and Aster scaber water extracts induce nitric oxide and cytokine production by lipopolysaccharide-activated macrophages. J. Med. Food 2009, 12, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chung, J.Y.; Lee, Y.J.; Park, S.; Kim, J.H.; Hahm, D.H.; Lee, H.J.; Shim, I. Effects of methanol extract of Uncariae Ramulus et Uncus on ibotenic acid-induced amnesia in the rat. J. Pharmacol. Sci. 2004, 96, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Dickens, M.; Rogers, J.S.; Cavanagh, J.; Raitano, A.; Xia, Z.; Halpern, J.R.; Greenberg, M.E.; Sawyers, C.L.; Davis, R.J. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 1997, 277, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; McCoy, M.K.; Frank-Cannon, T.C. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007, 208, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xu, Y.; Zhu, C.; Zhang, L.; Wu, A.; Yang, Y.; Xiong, Z.; Deng, C.; Huang, X.F.; Yenari, M.A.; et al. Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: The association with anti-inflammatory responses. PLoS ONE 2011, 6, e20945. [Google Scholar] [CrossRef] [PubMed]
- Knaryan, V.H.; Samantaray, S.; Park, S.; Azuma, M.; Inoue, J.; Banik, N.L. SNJ-1945, a calpain inhibitor, protects SH-SY5Y cells against MPP(+) and rotenone. J. Neurochem. 2014, 130, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, Z.; Bao, Q.; Zhang, Z.; Zhou, L.; Jiang, J.; Li, S. Protective effect of chinonin in MPTP-induced C57BL/6 mouse model of Parkinson’s disease. Biol. Pharm. Bull. 2014, 37, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Nath, C.; Shukla, R. The mechanism of action of MPTP-induced neuroinflammation and its modulation by melatonin in rat astrocytoma cells, C6. Free Radic. Res. 2010, 44, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Zhang, Y.; Lin, J.; Song, L.; Wang, X.; Liu, Z. Partial Depletion of Peripheral M1 Macrophages Reverses Motor Deficits in MPTP-Treated Mouse by Suppressing Neuroinflammation and Dopaminergic Neurodegeneration. Front. Aging Neurosci. 2018, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Dehmer, T.; Heneka, M.T.; Sastre, M.; Dichgans, J.; Schulz, J.B. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J. Neurochem. 2004, 88, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Li, T.J.; Qiu, Y.; Mao, J.Q.; Yang, P.Y.; Rui, Y.C.; Chen, W.S. Protective effects of Guizhi-Fuling-Capsules on rat brain ischemia/reperfusion injury. J. Pharmacol. Sci. 2007, 105, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Koppula, S.; Kumar, H.; Park, J.Y.; Kim, I.W.; More, S.V.; Kim, I.S.; Han, S.D.; Kim, S.K.; Yoon, S.H.; et al. α-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology 2015, 97, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Lee, H.H.; Han, M.H.; Kim, G.Y.; Hong, S.H.; Park, C.; Choi, Y.H. Ethanol extract of Poria cocos reduces the production of inflammatory mediators by suppressing the NF-kappaB signaling pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Complement. Altern. Med. 2014, 14, 101. [Google Scholar] [CrossRef] [Green Version]
- Janhom, P.; Dharmasaroja, P. Neuroprotective Effects of Alpha-Mangostin on MPP+-Induced Apoptotic Cell Death in Neuroblastoma SH-SY5Y Cells. J. Toxicol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Perier, C.; Bové, J.; Vila, M. Mitochondria and programmed cell death in Parkinson’s disease: Apoptosis and beyond. Antioxid. Redox Signal. 2017, 16, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.; Benabid, A.L.; Sadoul, R.; Verna, J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Tang, T.; Wu, H.J.; You, W.H.; Luo, J.K.; Lin, Y.; Liang, Q.H.; Li, X.Q.; Huang, X.; Yang, Q.D. Salvianolic acid B protects SH-SY5Y neuroblastoma cells from 1-methyl-4-phenylpyridinium-induced apoptosis. Biol. Pharm. Bull. 2010, 33, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Turmel, H.; Hartmann, A.; Parain, K.; Douhou, A.; Srinivasan, A.; Agid, Y.; Hirsch, E.C. Caspase-3 activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Mov. Disord. 2001, 16, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Kanthasamy, A.G.; Reddy, M.B. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology 2008, 245, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Guo, D.; Chen, B. Neuroprotective effects of astragaloside IV on Parkinson disease models of mice and primary astrocytes. Exp. Ther Med. 2017, 14, 5569–5575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.S.; Kim, Y.J.; Kim, B.Y.; Park, G.; Jeong, S.J. The Anti-neuroinflammatory Activity of Tectorigenin Pretreatment via Downregulated NF-κB and ERK/JNK Pathways in BV-2 Microglial and Microglia Inactivation in Mice with Lipopolysaccharide. Front. Pharmacol. 2018, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Kosaka, T.; Kakimura, J.I.; Matsuoka, Y.; Kohno, Y.; Nomura, Y.; Taniguchi, T. Protective effects of the antiparkinsonian drugs talipexole and pramipexole against 1-methyl-4-phenylpyridinium-induced apoptotic death in human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 1998, 54, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Doo, A.R.; Kim, S.N.; Kim, S.T.; Park, J.Y.; Chung, S.H.; Choe, B.Y.; Chae, Y.; Lee, H.; Yin, C.S.; Park, H.J. Bee venom protects SH-SY5Y human neuroblastoma cells from 1-methyl-4-phenylpyridinium-induced apoptotic cell death. Brain Res. 2012, 1429, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Kim, H.G.; Ju, M.S.; Choi, J.G.; Jeong, S.Y.; Oh, M.S. Effects of the hook of Uncaria rhynchophylla on neurotoxicity in the 6-hydroxydopamine model of Parkinson’s disease. J. Ethnopharmacol. 2009, 126, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Xiong, Y.; Sun, J.; Chen, C.; Gao, J.; Xu, H. Asiatic acid prevents oxidative stress and apoptosis by inhibiting the translocation of α-Synuclein into mitochondria. Front. Neurosci. 2018, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yu, H.; Huang, C.; Zhong, Q.; Chen, Y.; Xie, J.; Zhou, Z.; Xu, J.; Wang, H. Inhibition of phosphodiesterase 4 by FCPR16 protects SH-SY5Y cells against MPP+-induced decline of mitochondrial membrane potential and oxidative stress. Redox Biol. 2018, 16, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Jain, P.D.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience 2014, 277, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Jiang, M.; Cai, Q.; Fang, J.; Jin, L. MANF improves the MPP+/MPTP-induced Parkinson’s disease via improvement of mitochondrial function and inhibition of oxidative stress. Am. J. Transl Res. 2018, 10, 1284–1294. [Google Scholar] [PubMed]
- Li, L.; Shi, L.; Liu, H.; Luo, Q.; Huang, C.; Liu, W.; Chen, X.; Zeng, W.; Chen, Z. Changes in blood anti-oxidation enzyme levels in MPTP-treated monkeys. Neurosci. Lett. 2017, 649, 93–99. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Kim, I.S.; Koppula, S.; Kim, B.W.; Park, P.J.; Lim, B.O.; Choi, W.S.; Lee, K.H.; Choi, D.K. Protective effects of Gastrodia elata Blume on MPP+-induced cytotoxicity in human dopaminergic SH-SY5Y cells. J. Ethnopharmacol. 2010, 130, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Gao, L.; Wang, X.; Ding, X.; Teng, J. Pretreatment of glial cell-derived neurotrophic factor and geranylgeranylacetone ameliorates brain injury in Parkinson’s disease by its anti-apoptotic and anti-oxidative property. J. Cell. Biochem. 2018, 119, 5491–5502. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Chang, F.R.; Lo, Y.C. The Chinese herbal formula Liuwei dihuang protects dopaminergic neurons against Parkinson’s toxin through enhancing antioxidative defense and preventing apoptotic death. Phytomedicine 2014, 21, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Hu, J.H.; Wang, L.; Kim, H.S.; Jin, C.W.; Cho, D.H. Antioxidant and Anti-diabetes Activity of Extracts from Machilus thunbergii S. et Z. Korean J. Med. Crop Sci. 2010, 18, 34–39. [Google Scholar]
- Cao, Q.; Qin, L.; Huang, F.; Wang, X.; Yang, L.; Shi, H.; Wu, H.; Zhang, B.; Chen, Z.; Wu, X. Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways. Toxicol. Appl. Pharmacol. 2017, 319, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zheng, W.; Xin, N.; Chi, Z.H.; Wang, N.Q.; Nie, Y.X.; Feng, W.Y.; Wang, Z.Y. Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuv. Res. 2010, 13, 55–64. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Hepad 1 and Hepad 2 are available from the authors. |








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.H.; Kim, G.-J.; Lee, K.J.; Shin, J.S.; Kim, D.-H.; Park, B.-J.; An, J.H. Mitigation Effects of a Novel Herbal Medicine, Hepad, on Neuroinflammation, Neuroapoptosis, and Neuro-Oxidation. Molecules 2018, 23, 2920. https://doi.org/10.3390/molecules23112920
Song DH, Kim G-J, Lee KJ, Shin JS, Kim D-H, Park B-J, An JH. Mitigation Effects of a Novel Herbal Medicine, Hepad, on Neuroinflammation, Neuroapoptosis, and Neuro-Oxidation. Molecules. 2018; 23(11):2920. https://doi.org/10.3390/molecules23112920
Chicago/Turabian StyleSong, Da Hye, Gyeong-Ji Kim, Kwon Jai Lee, Jae Soo Shin, Dong-Hee Kim, Byung-Jun Park, and Jeung Hee An. 2018. "Mitigation Effects of a Novel Herbal Medicine, Hepad, on Neuroinflammation, Neuroapoptosis, and Neuro-Oxidation" Molecules 23, no. 11: 2920. https://doi.org/10.3390/molecules23112920
APA StyleSong, D. H., Kim, G. -J., Lee, K. J., Shin, J. S., Kim, D. -H., Park, B. -J., & An, J. H. (2018). Mitigation Effects of a Novel Herbal Medicine, Hepad, on Neuroinflammation, Neuroapoptosis, and Neuro-Oxidation. Molecules, 23(11), 2920. https://doi.org/10.3390/molecules23112920