A Simple and Efficient Synthesis of Highly Substituted Indeno[1,2-b]pyrrole and Acenaphtho[1,2-b]pyrrole Derivatives by Tandem Three-Component Reactions
Abstract
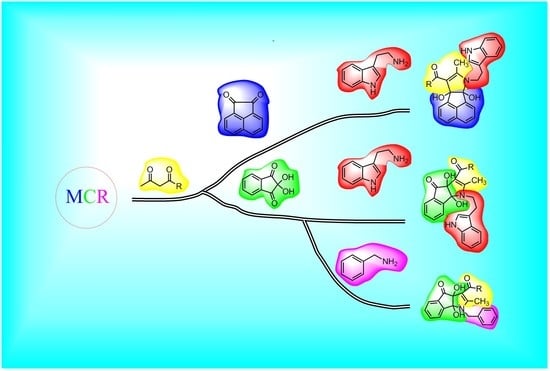
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Compounds 4, 6 and 8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fürstner, A. Chemistry and biology of roseophilin and the prodigiosin alkaloids: A survey of the last 2500 years. Angew. Chem. Int. Ed. Engl. 2003, 42, 3582–3603. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A.; Szillat, H.; Gabor, B.; Mynott, R. Platinum- and acid-catalyzed enyne metathesis reactions: Mechanistic studies and applications to the syntheses of streptorubin B and metacycloprodigiosin. J. Am. Chem. Soc. 1998, 120, 8305–8314. [Google Scholar] [CrossRef]
- Lipshutz, B.H. Five-membered heteroaromatic rings as intermediates in organic synthesis. Chem. Rev. 1986, 86, 795–819. [Google Scholar] [CrossRef]
- Balme, G. Pyrrole syntheses by multicomponent coupling reactions. Angew. Chem. Int. Ed. 2004, 43, 6238–6241. [Google Scholar] [CrossRef] [PubMed]
- Lehuédé, J.; Fauconneau, B.; Barrier, L.; Ourakow, M.; Piriou, A.; Vierfond, J. Synthesis and antioxidant activity of new tetraarylpyrroles. Eur. J. Med. Chem. 1999, 34, 991–996. [Google Scholar] [CrossRef]
- Bürli, R.W.; Jones, P.; McMinn, D.; Le, Q.; Duan, J.X.; Kaizerman, J.A.; Difuntorum, S.; Moser, H.E. DNA binding ligands targeting drug-resistant Gram-positive bacteria. Part 2: C-terminal benzimidazoles and derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Denny, W.A.; Rewcastle, G.W.; Baguley, B.C. Potential antitumor agents. 59. Structure-activity relationships for 2-phenylbenzimidazole-4-carboxamides, a new class of "minimal" DNA-intercalating agents which may not act via topoisomerase II. J. Med. Chem. 1990, 33, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, J.; Li, G.; Liu, Y. Diverse reactivity of zirconacyclocumulenes derived from coupling of benzynezirconocenes with 1,3-butadiynes towards acyl cyanides: Synthesis of indeno[2,1-b]pyrroles or [3]cumulenones. Angew. Chem. Int. Ed. 2009, 48, 5500–5504. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Meng, X.; Zhu, F.; Cheng, J.; Shao, X.; Li, Z. Tetrahydroindeno[1′,2′:4,5]pyrrolo[1,2-a]imidazol-5(1H)-ones as novel neonicotinoid insecticides: Reaction selectivity and substituent effects on the activity level. J. Agric. Food. Chem. 2015, 63, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Jabor, G.G.; Bouaziz, Z.; Winter, E.; Daflon-Yunes, N.; Aichele, D.; Nacereddine, A.; Marminon, C.; Valdameri, G.; Zeinyeh, W.; Bollacke, A.; et al. Converting potent indeno[1,2-b]indole inhibitors of protein kinase CK2 into selective inhibitors of the breast cancer resistance protein ABCG2. J. Med. Chem. 2015, 58, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, G.; Gao, J.; Song, T. Inclusion complex of a Bcl-2 inhibitor with cyclodextrin: Characterization, cellular accumulation, and in vivo antitumor activity. Mol. Pharm. 2010, 7, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, G.; Xie, F.; Song, T.; Chang, X. 3-Thiomorpholin-8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile (S1) based molecules as potent, dual inhibitors of B-cell lymphoma 2 (Bcl-2) and myeloid cell leukemia sequence 1 (Mcl-1): Structure-based design and structure-activity relationship studies. J. Med. Chem. 2011, 54, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, H.; Wu, G.; Li, Z.; Song, T.; Li, X.Q. Probing the difference between BH3 groove of Mcl-1 and Bcl-2 protein: Implications for dual inhibitors design. Eur. J. Med. Chem. 2011, 46, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Li, X.; Chang, X.; Liang, X.; Zhao, Y.; Wu, G.; Xie, S.; Su, P.; Wu, Z.; Feng, Y.; Zhang, Z. 3-Thiomorpholin-8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile (S1)derivatives as pan-Bcl-2-inhibitors of Bcl-2, Bcl-xL and Mcl-1. Bioorg. Med. Chem. 2013, 21, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 2000, 287, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.A. Recent applications of polymer-supported reagents and scavengers in combinatorial, parallel, or multistep synthesis. Curr. Opin. Chem. Biol. 2000, 4, 324–337. [Google Scholar] [CrossRef]
- Lu, L.Q.; Chen, J.R.; Xiao, W.J. Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 2012, 45, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, H.; Xu, P.F. Asymmetric catalytic cascade reactions for constructing diverse scaffolds and complex molecules. Acc. Chem. Res. 2015, 48, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Domling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Orru, R.V.A.; Degreef, M. Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 2003, 10, 1471–1499. [Google Scholar] [CrossRef]
- Hulme, C.; Gore, V. “Multi-component reactions: Emerging chemistry in drug discovery” from xylocain to crixivan. Curr. Med. Chem. 2003, 10, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.M.; Zhu, J.S.; Tantillo, D.J.; Kurth, M.J. Multicomponent assembly of highly substituted indoles by dual palladium-catalyzed coupling reactions. Angew. Chem. Int. Ed. 2012, 51, 10588–10591. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.Y.; Cai, C. Iodine catalyzed one-pot multicomponent synthesis of a library of compounds containing tetrazolo[1,5-a]pyrimidine core. J. Comb. Chem. 2010, 12, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Maximizing synthetic efficiency: Multi-component transformations lead the way. Eur. J. Chem. 2000, 6, 3321–3329. [Google Scholar] [CrossRef]
- Kumaravel, K.; Vasuki, G. Multi-component reactions in water. Curr. Org. Chem. 2009, 13, 1820–1841. [Google Scholar] [CrossRef]
- Koopmanschap, G.; Ruijter, E.; Orru, R.V. Isocyanide-based multicomponent reactions towards cyclic constrained peptidomimetics. Beilstein. J. Org. Chem. 2014, 10, 544–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Kim, D.B.; Domling, A. Cyanoacetamide MCR (III): Three-component Gewald reactions revisited. J. Comb. Chem. 2010, 12, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Cen, L.Q.; Ma, Y.; Wang, J.; Zhu, S.L. One-pot, five-component 1,3-dipolar cycloaddition: A facile synthesis of spiropyrrolidine and spiropyrrolizidine derivatives. Tetrahedron. Lett. 2018, 59, 1686–1690. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Liu, H.Z.; Xu, Z.; Zhu, S.L. A Facile and Efficient Synthesis of Spiro[indoline-3,5′-pyrido [2,3-d]pyrimidine] Derivatives via Microwave-assisted Multicomponent Reactions. Lett. Org. Chem. 2015, 12, 62–66. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Xu, Z.; Zhu, S. Combinatorial synthesis of functionalized spirooxindole-pyrrolidine/pyrrolizidine/pyrrolothiazole derivatives via three-component 1,3-dipolar cycloaddition reactions. ACS Comb. Sci. 2014, 16, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.L.; Wang, J.; Xu, Z.; Li, J. An efficient one-pot synthesis of pyrano[3,2-c]quinolin-2,5-dione derivatives catalyzed by l-proline. Molecules 2012, 17, 13856–13863. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |








| Entry | Solvent | Temp (°C) | Time (h) | Yield (%) a |
|---|---|---|---|---|
| 1 | Methanol | r.t. | 3 | 57 |
| 2 | Chloroform | r.t. | 3 | 40 |
| 3 | Acetonitrile | r.t. | 3 | 45 |
| 4 | Toluene | r.t. | 3 | 35 |
| 5 | Water | r.t. | 3 | 31 |
| 7 | Ethanol | r.t. | 3 | 85 |
| 8 | Ethanol | 40 | 3 | 83 |
| 9 | Ethanol | reflux | 3 | 84 |
| Entry | Product | Structure | Time (h) | Yield (%) a | m.p./(°C) |
|---|---|---|---|---|---|
| 1 | 4a |  | 3 | 85 | 149–151 |
| 2 | 4b |  | 2.5 | 93 | 121–123 |
| 3 | 4c |  | 2.5 | 92 | 101–103 |
| 4 | 4d |  | 3 | 91 | 110–112 |
| 5 | 4e |  | 3.5 | 94 | 100–102 |
| 6 | 4f |  | 2.5 | 75 | 164–166 |
| Entry | Product | Structure | Time (h) | Yield (%) a | m.p./(°C) |
|---|---|---|---|---|---|
| 1 | 6a |  | 3 | 72 | 161–163 |
| 2 | 6b |  | 3 | 83 | 145–147 |
| 3 | 6c |  | 2.5 | 78 | 161–163 |
| 4 | 6d |  | 2.5 | 70 | 112–114 |
| 5 | 6e |  | 3.5 | 85 | 95–97 |
| Entry | Product | Structure | Time (h) | Yield (%) a | m.p./(°C) |
|---|---|---|---|---|---|
| 1 | 8a |  | 2.5 | 75 | 154–156 |
| 2 | 8b |  | 3 | 71 | 72–73 |
| 3 | 8c |  | 2.5 | 83 | 71–73 |
| 4 | 8d |  | 3.5 | 88 | 83–85 |
| 5 | 8e |  | 4 | 88 | 70–71 |
| 6 | 8f |  | 2.5 | 81 | 175–177 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Zhu, S.; Ma, Y.; Wen, R.; Cen, L.; Gong, P.; Wang, J. A Simple and Efficient Synthesis of Highly Substituted Indeno[1,2-b]pyrrole and Acenaphtho[1,2-b]pyrrole Derivatives by Tandem Three-Component Reactions. Molecules 2018, 23, 3031. https://doi.org/10.3390/molecules23113031
Tang X, Zhu S, Ma Y, Wen R, Cen L, Gong P, Wang J. A Simple and Efficient Synthesis of Highly Substituted Indeno[1,2-b]pyrrole and Acenaphtho[1,2-b]pyrrole Derivatives by Tandem Three-Component Reactions. Molecules. 2018; 23(11):3031. https://doi.org/10.3390/molecules23113031
Chicago/Turabian StyleTang, Xiaodong, Songlei Zhu, Ying Ma, Ren Wen, Lanqi Cen, Panwei Gong, and Jing Wang. 2018. "A Simple and Efficient Synthesis of Highly Substituted Indeno[1,2-b]pyrrole and Acenaphtho[1,2-b]pyrrole Derivatives by Tandem Three-Component Reactions" Molecules 23, no. 11: 3031. https://doi.org/10.3390/molecules23113031
APA StyleTang, X., Zhu, S., Ma, Y., Wen, R., Cen, L., Gong, P., & Wang, J. (2018). A Simple and Efficient Synthesis of Highly Substituted Indeno[1,2-b]pyrrole and Acenaphtho[1,2-b]pyrrole Derivatives by Tandem Three-Component Reactions. Molecules, 23(11), 3031. https://doi.org/10.3390/molecules23113031