Agar Extraction By-Products from Gelidium sesquipedale as a Source of Glycerol-Galactosides
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
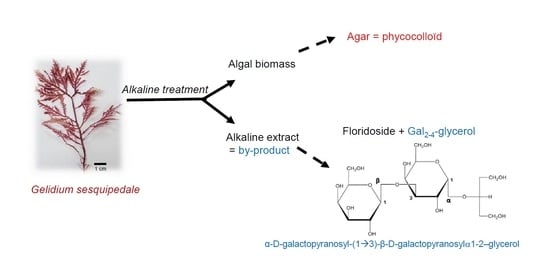
4.1. Raw Material
4.2. Alkali Treatment
4.3. Fractionation of the Alkaline Extract
4.4. Electrospray Mass Spectrometry Analyses
4.5. 1H-Nuclear Magnetic Resonance (NMR) Experiments
4.6. Determination of the Glycosidic Linkage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marinho-Soriano, E.; Bourret, E. Polysaccharides from the red seaweed Gracilaria dura (Gracilariales, Rhodophyta). Bioresour. Technol. 2005, 96, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y. Relation between structure and rheological/thermal properties of agar. A mini-review on the effect of alkali treatment and the role of agaropectin. Food Struct. 2017, 13, 24–34. [Google Scholar] [CrossRef]
- Herrero, M.; Sánchez-Camargo, A.D.P.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Cheong, K.-L.; Qiu, H.-M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides Derived from Red Seaweed: Production, Properties, and Potential Health and Cosmetic Applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef] [PubMed]
- Eggert, A.; Karsten, U. Low molecular weight carbohydrates in red algae—an ecophysiological and biochemical perspective. In Red Algae in the Genomic Age, Cellular Origin, Life in Extreme Habitats, and Astrobiology; Seckbach, J., Chapman, D., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 13, pp. 443–456. ISBN 978-90-481-3795-4. [Google Scholar]
- Li, S.-Y.; Shabtai, Y.; Arad, S. Floridoside as a carbon precursor for the synthesis of cell-wall polysaccharide in the red microalga Porphyridium sp. (Rhodophyta). J. Phycol. 2002, 38, 931–938. [Google Scholar] [CrossRef]
- Majak, W.; Craigie, J.S.; McLachlan, J. Photosynthesis in algae–accumulation products in Rhodophyceae. Can. J. Bot. 1966, 44, 541–549. [Google Scholar] [CrossRef]
- Kim, M.; Li, Y.X.; Dewapriya, P.; Ryu, B.; Kim, S.K. Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia. BMB Rep. 2013, 46, 398–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, K.; Zhang, X.-H. Quorum quenching agents: Resources for antivirulence therapy. Mar. Drugs 2014, 12, 3245–3282. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, J.; Yan, X.; Yang, R.; Zhang, C.; Chen, H. Extraction of floridoside and the effect of floridoside on Ctenopharyngodon idella surimi during frozen storage. J. Fish. China 2017, 41, 311–318. [Google Scholar] [CrossRef]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive compounds from macroalgae in the new millennium: Implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef]
- Li, Y.-X.; Li, Y.; Lee, S.-H.; Qian, Z.-J.; Kim, S.-K. Inhibitors of oxidation and matrix metalloproteinases, floridoside, and D-isofloridoside from marine red alga Laurencia undulata. J. Agric. Food Chem. 2010, 58, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Li, Y.-X.; Kang, K.-H.; Kim, S.-K.; Kim, D.G. Floridoside from Laurencia undulata promotes osteogenic differentiation in murine bone marrow mesenchymal cells. J. Funct. Foods 2015, 19, 505–511. [Google Scholar] [CrossRef]
- Karsten, U.; Michalik, D.; Michalik, M.; West, J.A. A new unusual low molecular weight carbohydrate in the red algal genus Hypoglossum (Delesseriaceae, Ceramiales) and its possible function as an osmolyte. Planta 2005, 222, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, D.; Luo, Q.; Mou, T.; Yang, R.; Chen, H.; He, S.; Yan, X. Determination of floridoside and isofloridoside in red algae by high-performance liquid chromatography–tandem mass spectrometry. Anal. Lett. 2014, 47, 2307–2316. [Google Scholar] [CrossRef]
- Obando, C.Z.; Linossier, I.; Kervarec, N.; Zubia, M.; Turquet, J.; Faÿ, F.; Rehel, K. Rapid identification of osmolytes in tropical microalgae and cyanobacteria by 1H HR-MAS NMR spectroscopy. Talanta 2016, 153, 372–380. [Google Scholar] [CrossRef]
- Kono, H.; Kondo, N.; Hirabayashi, K.; Ogata, M.; Totani, K.; Ikematsu, S.; Osada, M. NMR spectroscopic structural characterization of a water-soluble β-(1->3, 1->6)-glucan from Aureobasidium pullulans. Carbohydr. Polym. 2017, 174, 876–886. [Google Scholar] [CrossRef]
- Kienteka, S.S.; Corrêa-Ferreira, M.L.; de Oliveira Petkowicz, C.L. Characterization of cell wall polysaccharides from Sicana odorifera fruit and structural analysis of a galactan-rich fraction pectins as side chains. Carbohydr. Polym. 2018, 197, 395–402. [Google Scholar] [CrossRef]
- Sakugawa, H.; Handa, N.; Yagi, K. Distribution of glycosylglycerols and oligosaccharides in the marine environment and their ecological significance in the deep sea. Mar. Biol. 1990, 106, 309–313. [Google Scholar] [CrossRef]
- Benson, A.A.; Wiser, R.; Ferrari, R.A.; Miller, J.A. Photosynthesis of galactolipids. J. Am. Chem. Soc. 1958, 80, 4740. [Google Scholar] [CrossRef]
- Doehlert, D.C.; Moreau, R.A.; Welti, R.; Roth, M.R.; McMullen, M.S. Polar lipids from oat kernels. Cereal Chem. 2010, 87, 467–474. [Google Scholar] [CrossRef]
- Deslandes, E.; Bodeau, C. Cosmetic Composition Containing Red Algae Extract Comprising a Combination of Floridoside and Isethionic Acid. European Patent 1743628, 17 January 2007. [Google Scholar]
- Lebbar, S.; Faugeron-Girard, C.; Gloaguen, V. Utilisation d’un extrait ou d’une fraction d’extrait d’algue rouge agarophyte comme éliciteur/stimulateur de défense végétal et application dudit extrait ou de ladite fraction d’extrait. French Patent deposited under registration number 1870542, 7 May 2018. [Google Scholar]
- Buffetto, F.; Cornuault, V.; Rydahl, M.G.; Ropartz, D.; Alvarado, C.; Echasserieau, V.; Le Gall, S.; Bouchet, B.; Tranquet, O.; Verhertbruggen, Y.; et al. The deconstruction of pectic rhamnogalacturonan I unmasks the occurrence of a novel arabinogalactan oligosaccharide epitope. Plant Cell Physiol. 2015, 56, 2181–2196. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the alkaline extract are not available from the authors. |





| Linked Sugar | % Total Sugar (Mean) | Standard Deviation |
|---|---|---|
| t-Gal | 88.0 | 2.6 |
| (1,3)-Gal | 6.2 | 0.7 |
| (1,4)-Gal | 5.7 | 1.9 |
| Compounds | Molecular Weight |
|---|---|
| Isethionic acid | 126 |
| 3,6-Anhydrogalactose | 162 |
| Hexose | 180 |
| Floridoside (Gal-glycerol) | 254 |
| Disaccharide (hexose) | 342 |
| Gal2-glycerol | 416 |
| Trisaccharide (hexose) | 504 |
| Gal3-glycerol | 578 |
| Disaccharide (hexose) | 666 |
| Gal4-glycerol | 740 |
| Yield (algal dry mass) | 8.4% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebbar, S.; Fanuel, M.; Le Gall, S.; Falourd, X.; Ropartz, D.; Bressollier, P.; Gloaguen, V.; Faugeron-Girard, C. Agar Extraction By-Products from Gelidium sesquipedale as a Source of Glycerol-Galactosides. Molecules 2018, 23, 3364. https://doi.org/10.3390/molecules23123364
Lebbar S, Fanuel M, Le Gall S, Falourd X, Ropartz D, Bressollier P, Gloaguen V, Faugeron-Girard C. Agar Extraction By-Products from Gelidium sesquipedale as a Source of Glycerol-Galactosides. Molecules. 2018; 23(12):3364. https://doi.org/10.3390/molecules23123364
Chicago/Turabian StyleLebbar, Salim, Mathieu Fanuel, Sophie Le Gall, Xavier Falourd, David Ropartz, Philippe Bressollier, Vincent Gloaguen, and Céline Faugeron-Girard. 2018. "Agar Extraction By-Products from Gelidium sesquipedale as a Source of Glycerol-Galactosides" Molecules 23, no. 12: 3364. https://doi.org/10.3390/molecules23123364
APA StyleLebbar, S., Fanuel, M., Le Gall, S., Falourd, X., Ropartz, D., Bressollier, P., Gloaguen, V., & Faugeron-Girard, C. (2018). Agar Extraction By-Products from Gelidium sesquipedale as a Source of Glycerol-Galactosides. Molecules, 23(12), 3364. https://doi.org/10.3390/molecules23123364