Identification of Pinocembrin as an Anti-Glycation Agent and α-Glucosidase Inhibitor from Fingerroot (Boesenbergia rotunda): The Tentative Structure–Activity Relationship towards MG-Trapping Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Plant Isolation
2.2. Glycation Inhibition
2.2.1. Effect of Isolated Compounds on Fluorescent-AGEs Formation Activity by an MG-BSA Assay
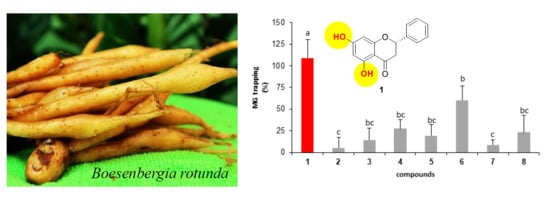
2.2.2. Effect of Isolated Compounds on MG-Trapping Activity
- The presence of a methoxy group on the aromatic ring A of flavanone.
- The positions of methoxy groups on the aromatic ring A of flavanone.
- The ring C structure of flavanone and the α-β unsaturated ketone structure of chalcone.
- The presence of a geranyl group on chalcone.
- The positions of geranyl groups on ring A (chalcone), or at α-β unsaturated ketone (dihydrochalcone).
- The positions of the methoxy group on the aromatic ring A of dihydrochalcone.
2.3. α-Glucosidase Inhibitory Activity and Kinetic Study of Pinocembrin (1)
3. Material and Method
3.1. Plant Material and Isolation
3.2. In Vitro Glycation of Bovine Serum Albumin (BSA) by MG-BSA Assay
3.3. Determination of Direct MG-Trapping Activity by HPLC
3.4. α-Glucosidase Inhibitory Activity
3.5. Kinetic Study of α-Glucosidase Inhibition
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goh, S.G.K.; Rusli, B.N.; Khalid, B.A.K. Evolution of diabetes management in the 21st century: The contribution of quality of life measurement in asians. Asia Pac. J. Clin. Nutr. 2015, 24, 190–198. [Google Scholar] [PubMed]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Lapolla, A.; Flamini, R.; Dalla Vedova, A.; Senesi, A.; Reitano, R.; Fedele, D.; Basso, E.; Seraglia, R.; Traldi, P. Glyoxal and methylglyoxal levels in diabetic patients: Quantitative determination by a new gc/ms method. Clin. Chem. Lab. Med. 2003, 41, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; Wu, C.-H.; Yen, G.-C. Perspective of advanced glycation end products on human health. J. Agric. Food Chem. 2018, 66, 2065–2070. [Google Scholar] [CrossRef]
- Odani, H.; Shinzato, T.; Matsumoto, Y.; Usami, J.; Maeda, K. Increase in three α,β-dicarbonyl compound levels in human uremic plasma: Specificin vivodetermination of intermediates in advanced maillard reaction. Biochem. Biophys. Res. Commun. 1999, 256, 89–93. [Google Scholar] [CrossRef]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Price, C.L.; Knight, S.C. Advanced glycation: A novel outlook on atherosclerosis. Curr. Pharm. Des. 2007, 13, 3681–3687. [Google Scholar] [CrossRef]
- Richard, J.P. Mechanism for the formation of methylglyoxal from triosephosphates. Biochem. Soc. Trans. 1993, 21, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Freedman, B.I.; Wuerth, J.-P.; Cartwright, K.; Bain, R.P.; Dippe, S.; Hershon, K.; Mooradian, A.D.; Spinowitz, B.S. Design and baseline characteristics for the aminoguanidine clinical trial in overt type 2 diabetic nephropathy (action ii). Controlled Clin. Trials 1999, 20, 493–510. [Google Scholar] [CrossRef]
- Elosta, A.; Ghous, T.; Ahmed, N. Natural products as anti-glycation agents: Possible therapeutic potential for diabetic complications. Curr. Diabetes Rev. 2012, 8, 92–108. [Google Scholar] [CrossRef]
- Eng-Chong, T.; Yean-Kee, L.; Chin-Fei, C.; Choon-Han, H.; Sher-Ming, W.; Li-Ping, C.T.; Gen-Teck, F.; Khalid, N.; Abd Rahman, N.; Karsani, S.A.; et al. Boesenbergia rotunda: From ethnomedicine to drug discovery. J. Evid.-Based Complement. Altern. Med. 2012, 2012, 473637. [Google Scholar] [CrossRef] [PubMed]
- Ongwisespaiboon, O.; Jiraungkoorskul, W. Fingerroot, boesenbergia rotunda and its aphrodisiac activity. Pharmacogn. Rev. 2017, 11, 27–30. [Google Scholar] [PubMed]
- Ma, H.; Liu, W.; Frost, L.; Kirschenbaum, L.J.; Dain, J.A.; Seeram, N.P. Glucitol-core containing gallotannins inhibit the formation of advanced glycation end-products mediated by their antioxidant potential. Food Funct. 2016, 7, 2213–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sompong, W.; Adisakwattana, S. Inhibitory effect of herbal medicines and their trapping abilities against methylglyoxal-derived advanced glycation end-products. BMC Complementary Altern. Med. 2015, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Sompong, W.; Cheng, H.; Adisakwattana, S. Ferulic acid prevents methylglyoxal-induced protein glycation, DNA damage, and apoptosis in pancreatic β-cells. J. Physiol. Biochem. 2017, 73, 121–131. [Google Scholar] [CrossRef]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef]
- Hwang, S.H.; Wang, Z.; Quispe, Y.N.G.; Lim, S.S.; Yu, J.M. Evaluation of aldose reductase, protein glycation, and antioxidant inhibitory activities of bioactive flavonoids in matricaria recutita l. And their structure-activity relationship. J. Diabetes Res. 2018, 2018, 3276162. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Wang, T.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg. Med. Chem. 2003, 11, 5317–5323. [Google Scholar] [CrossRef]
- Rezazadeh, S.; Ebrahimi, A.; Nowroozi, A. The effects of structural properties on the methylglyoxal scavenging mechanism of flavonoid aglycones: A quantum mechanical study. Comput. Theor. Chem. 2017, 1118, 26–38. [Google Scholar] [CrossRef]
- Shao, X.; Chen, H.; Zhu, Y.; Sedighi, R.; Ho, C.-T.; Sang, S. Essential structural requirements and additive effects for flavonoids to scavenge methylglyoxal. J. Agric. Food Chem. 2014, 62, 3202–3210. [Google Scholar] [CrossRef]
- Jaipetch, T.; Kanghae, S.; Pancharoen, O.; Patrick, V.A.; Reutrakul, V.; Tuntiwachwuttikul, P.; White, A.H. Constituents of boesenbergia pandurata (syn. Kaempferia pandurata): Isolation, crystal structure and synthesis of (±)-boesenbergin a. Aust. J. Chem. 1982, 35, 351–361. [Google Scholar] [CrossRef]
- Itokawa, H.; Morita, M.; Mihashi, S. Phenolic compounds from the rhizomes of alpinia speciosa. Phytochemistry 1981, 20, 2503–2506. [Google Scholar] [CrossRef]
- Ranjith, W.; Dharmaratne, H.; Dhammika Nanayakkara, N.P.; Khan, I.A. Kavalactones from piper methysticum, and their 13c nmr spectroscopic analyses. Phytochemistry 2002, 59, 429–433. [Google Scholar] [CrossRef]
- Pandji, C.; Grimm, C.; Wray, V.; Witte, L.; Proksch, P. Insecticidal constituents from four species of the zingiberaceae. Phytochemistry 1993, 34, 415–419. [Google Scholar] [CrossRef]
- Tuntiwachwuttikul, P.; Pancharoen, O.; Reutrakul, V.; Byrne, L.T. (1′rs, 2′sr, 6′rs)-(2, 6-dihydroxy-4-methoxyphenyl)-[3′-methyl-2′-(3″-methylbut-2″-enyl)-6′-phenyl-cyclohex-3′-enyllmethanone (panduratin a)- a constituent of the red rhizomes of a variety of boesenbergia pandurata. Aust. J. Chem. 1984, 37, 449–453. [Google Scholar] [CrossRef]
- Ramadhan, R.; Phuwapraisirisan, P. New arylalkanones from horsfieldia macrobotrys, effective antidiabetic agents concomitantly inhibiting α-glucosidase and free radicals. Bioorg. Med. Chem. Lett. 2015, 25, 4529–4533. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–8 are available from the authors. |











| Activity | MG-Trapping | |
|---|---|---|
| Correlation Coefficient | p-Value (2-Tailed) | |
| AGEs formation inhibition | 0.159 | 0.707 |
| Enzyme | Ki (µM) | Ki′ (µM) | Inhibition Type |
|---|---|---|---|
| Maltase | 93 | 138 | Mixed |
| Sucrase | 51 | 253 | Mixed |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potipiranun, T.; Adisakwattana, S.; Worawalai, W.; Ramadhan, R.; Phuwapraisirisan, P. Identification of Pinocembrin as an Anti-Glycation Agent and α-Glucosidase Inhibitor from Fingerroot (Boesenbergia rotunda): The Tentative Structure–Activity Relationship towards MG-Trapping Activity. Molecules 2018, 23, 3365. https://doi.org/10.3390/molecules23123365
Potipiranun T, Adisakwattana S, Worawalai W, Ramadhan R, Phuwapraisirisan P. Identification of Pinocembrin as an Anti-Glycation Agent and α-Glucosidase Inhibitor from Fingerroot (Boesenbergia rotunda): The Tentative Structure–Activity Relationship towards MG-Trapping Activity. Molecules. 2018; 23(12):3365. https://doi.org/10.3390/molecules23123365
Chicago/Turabian StylePotipiranun, Thammatee, Sirichai Adisakwattana, Wisuttaya Worawalai, Rico Ramadhan, and Preecha Phuwapraisirisan. 2018. "Identification of Pinocembrin as an Anti-Glycation Agent and α-Glucosidase Inhibitor from Fingerroot (Boesenbergia rotunda): The Tentative Structure–Activity Relationship towards MG-Trapping Activity" Molecules 23, no. 12: 3365. https://doi.org/10.3390/molecules23123365
APA StylePotipiranun, T., Adisakwattana, S., Worawalai, W., Ramadhan, R., & Phuwapraisirisan, P. (2018). Identification of Pinocembrin as an Anti-Glycation Agent and α-Glucosidase Inhibitor from Fingerroot (Boesenbergia rotunda): The Tentative Structure–Activity Relationship towards MG-Trapping Activity. Molecules, 23(12), 3365. https://doi.org/10.3390/molecules23123365