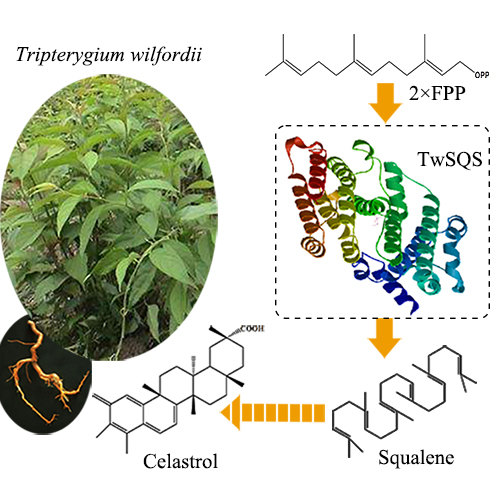
Cloning, Expression Analysis and Functional Characterization of Squalene Synthase (SQS) from Tripterygium wilfordii
Abstract
:1. Introduction
2. Results
2.1. Sequence Analysis of TwSQS
2.2. Purification and Identification of Recombinant Protein
2.3. Functional Characterization of the TwSQS
2.4. Expression Analysis of TwSQS
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Total RNA Extraction and cDNA Synthesis
4.3. Cloning and Bioinformatics Analyses of TwSQS
4.4. Expression of Recombinant Protein
4.5. Purification of Expressed TwSQS and Western Blot Analysis
4.6. In Vitro Enzyme Reaction
4.7. Expression Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abe, I.; Rohmer, M.; Prestwich, G.D. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem. Rev. 1993, 93, 2189–2206. [Google Scholar] [CrossRef]
- Brinker, A.M.; Ma, J.; Lipsky, P.E.; Raskin, I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 2007, 68, 732–766. [Google Scholar] [CrossRef] [PubMed]
- Brüning, R.; Wagner, H. Übersicht über die celastraceen-inhaltsstoffe: Chemie, chemotaxonomie, biosynthese, pharmakologie. Phytochemistry 1978, 17, 1821–1858. [Google Scholar] [CrossRef]
- Chou, T.; Mei, P. Study on Chinese herb Lei Gong, Teng Tripterygium wilfordii Hook f. I: The coloring substance and the sugars. Chin. J. Physiol. 1936, 10, 529–534. [Google Scholar]
- Wang, C.; Shi, C.; Yang, X.; Yang, M.; Sun, H.; Wang, C. Celastrol suppresses obesity process via increasing antioxidant capacity and improving lipid metabolism. Eur. J. Pharmacol. 2014, 744, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Pandey, M.K.; Aggarwal, B.B. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-κB–regulated gene products and TAK1-mediated NF-κB activation. Blood 2007, 109, 2727–2735. [Google Scholar] [PubMed]
- Youn, G.S.; Kwon, D.-J.; Ju, S.M.; Rhim, H.; Bae, Y.S.; Choi, S.Y.; Park, J. Celastrol ameliorates HIV-1 Tat-induced inflammatory responses via NF-kappaB and AP-1 inhibition and heme oxygenase-1 induction in astrocytes. Toxicol. Appl. Pharmacol. 2014, 280, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, D.; Cui, Q.C.; Yuan, X.; Dou, Q.P. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006, 66, 4758–4765. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, J.; Hernandez, M.A.S.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, L.; Alberobello, A.T.; Gavrilova, O.; Bagattin, A.; Skarulis, M.; Liu, J.; Finkel, T.; Mueller, E. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1α transcriptional axis. Cell Metab. 2015, 22, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Camelio, A.M.; Johnson, T.C.; Siegel, D. Total Synthesis of Celastrol, Development of a Platform to Access Celastroid Natural Products. J. Am. Chem. Soc. 2015, 137, 11864–11867. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Zhu, C.; Yang, Y.; Feng, M.; Ma, Z.; Feng, J.; Zhang, X. Elicitation and in situ adsorption enhanced secondary metabolites production of Tripterygium wilfordii Hook. f. adventitious root fragment liquid cultures in shake flask and a modified bubble column bioreactor. Bioprocess Biosyst. Eng. 2014, 37, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Haralampidis, K.; Trojanowska, M.; Osbourn, A.E. Biosynthesis of triterpenoid saponins in plants. In History and Trends in Bioprocessing and Biotransformation; Springer: Berlin, Germany, 2002; pp. 31–49. [Google Scholar]
- Gupta, N.; Sharma, P.; Kumar, R.S.; Vishwakarma, R.K.; Khan, B. Functional characterization and differential expression studies of squalene synthase from Withania somnifera. Mol. Biol. Rep. 2012, 39, 8803–8812. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.W.; Liang, W.Q.; Zhang, D.B.; Wang, N.; Wang, C.G.; Pan, Y.J. Cloning and characterization of squalene synthase (SQS) gene from Ganoderma lucidum. J. Microbiol. Biotechnol. 2007, 17, 1106–1112. [Google Scholar] [PubMed]
- Lee, S.; Poulter, C.D. Cloning, solubilization, and characterization of squalene synthase from Thermosynechococcus elongatus BP-1. J. Bacteriol. 2008, 190, 3808–3816. [Google Scholar] [CrossRef] [PubMed]
- Mckenzie, T.L.; Jiang, G.J.; Straubhaar, J.R.; Conrad, D.G.; Shechter, I. Molecular-cloning, expression, and characterization of the cDNA for the rat hepatic squalene synthase. J. Biol. Chem. 1992, 267, 21368–21374. [Google Scholar] [PubMed]
- Robinson, G.W.; Tsay, Y.H.; Kienzle, B.K.; Smithmonroy, C.A.; Bishop, R.W. Conservation between human and fungal squalene synthetases—similarities in structure, function, and regulation. Mol. Cell. Biol. 1993, 13, 2706–2717. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Karst, F.; Charles, A.D. Cloning, expression and characterization of the cDNA-encoding human hepatic squalene synthase, and its relationship to phytoene synthase. Gene 1993, 136, 185–192. [Google Scholar] [CrossRef]
- Busquets, A.; Keim, V.; Closa, M.; Del Arco, A.; Boronat, A.; Arró, M.; Ferrer, A. Arabidopsis thaliana contains a single gene encoding squalene synthase. Plant Mol. Biol. 2008, 67, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Liu, S.; Su, P.; Yuan, Y.; Huang, L. Cloning, prokaryotic expression and functional analysis of squalene synthase (SQS) in Magnolia officinalis. Protein Expr. Purif. 2016, 120, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Jiang, D.; Chen, Y.; Shen, Y.; Yuan, Q.; Lin, H.; Zha, L.; Zhang, Y.; Huang, L. Molecular cloning and functional analysis of squalene synthase 2 (SQS2) in Salvia miltiorrhiza Bunge. Front. Plant Sci. 2016, 7, 1274. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-D.; Han, J.-Y.; Huh, G.H.; Choi, Y.-E. Expression and functional characterization of three squalene synthase genes associated with saponin biosynthesis in Panax ginseng. Plant Cell Physiol. 2010, 52, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Rong, Q.; Chen, Y.; Yuan, Q.; Shen, Y.; Guo, J.; Yang, Y.; Zha, L.; Wu, H.; Huang, L. Molecular cloning and functional analysis of squalene synthase (SS) in Panax notoginseng. Int. J. Biol. Macromol. 2017, 95, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Neelakadan, A.K.; Quach, T.N.; Valliyodan, B.; Kumar, R.; Zhang, Z.; Nguyen, H.T. Molecular characterization of Glycine max squalene synthase genes in seed phytosterol biosynthesis. Plant Physiol. Biochem. 2013, 73, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Patel, P.; Kendurkar, S.V.; Thulasiram, H.V.; Khan, B.M. Overexpression of squalene synthase in Withania somnifera leads to enhanced withanolide biosynthesis. Plant Cell Tissue Organ Cult. 2015, 122, 409–420. [Google Scholar] [CrossRef]
- Lee, M.-H.; Jeong, J.-H.; Seo, J.-W.; Shin, C.-G.; Kim, Y.-S.; In, J.-G.; Yang, D.-C.; Yi, J.-S.; Choi, Y.-E. Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol. 2004, 45, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-W.; Jeong, J.-H.; Shin, C.-G.; Lo, S.-C.; Han, S.-S.; Yu, K.-W.; Harada, E.; Han, J.-Y.; Choi, Y.-E. Overexpression of squalene synthase in Eleutherococcus senticosus increases phytosterol and triterpene accumulation. Phytochemistry 2005, 66, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Cho, J.H.; Park, S.; Han, J.-Y.; Back, K.; Choi, Y.-E. Gene regulation patterns in triterpene biosynthetic pathway driven by overexpression of squalene synthase and methyl jasmonate elicitation in Bupleurum falcatum. Planta 2011, 233, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Ginzberg, I.; Thippeswamy, M.; Fogelman, E.; Demirel, U.; Mweetwa, A.M.; Tokuhisa, J.; Veilleux, R.E. Induction of potato steroidal glycoalkaloid biosynthetic pathway by overexpression of cDNA encoding primary metabolism HMG-CoA reductase and squalene synthase. Planta 2012, 235, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Devarenne, T.P.; Shin, D.H.; Back, K.; Yin, S.H.; Chappell, J. Molecular characterization of tobacco squalene synthase and regulation in response to fungal elicitor. Arch. Biochem. Biophys. 1998, 349, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.S.; Jiang, K.J.; Pi, Y.; Hou, R.; Liao, Z.H.; Cao, Y.; Han, X.; Wang, Q.; Sun, X.F.; Tang, K.X. Molecular cloning and characterization of the yew gene encoding squalene synthase from Taxus cuspidata. J. Biochem. Mol. Biol. 2007, 40, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huo, Y.-B.; Liu, Y.; Feng, J.-T.; Ma, Z.-Q.; Zhu, C.-S.; Zhang, X. Differential expressed analysis of Tripterygium wilfordii unigenes involved in terpenoid backbone biosynthesis. J. Asian Nat. Prod. Res. 2017, 19, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.D.; Ishii, Y.; Spencer, T.A.; Shechter, I. Function-structure studies and identification of three enzyme domains involved in the catalytic activity in rat hepatic squalene synthase. J. Biol. Chem. 1998, 273, 12515–12525. [Google Scholar] [CrossRef] [PubMed]
- Pandit, J.; Danley, D.E.; Schulte, G.K.; Mazzalupo, S.; Pauly, T.A.; Hayward, C.M.; Hamanaka, E.S.; Thompson, J.F.; Harwood, H.J. Crystal structure of human squalene synthase—A key enzyme in cholesterol biosynthesis. J. Biol. Chem. 2000, 275, 30610–30617. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Cao, X.; Li, C.; Chen, Y.; Yuan, B.; Xu, Y.; Jiang, J. Molecular cloning and expression analysis of a squalene synthase gene from a medicinal plant, Euphorbia pekinensis Rupr. Acta Physiol. Plant. 2013, 35, 3007–3014. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Yin, J.; Li, C.; Zhan, Y.; Xiao, J.; Liang, T.; Li, X. Molecular cloning and promoter analysis of squalene synthase and squalene epoxidase genes from Betula platyphylla. Protoplasma 2016, 253, 1347–1363. [Google Scholar] [CrossRef] [PubMed]
- Bhat, W.W.; Lattoo, S.K.; Razdan, S.; Dhar, N.; Rana, S.; Dhar, R.S.; Khan, S.; Vishwakarma, R.A. Molecular cloning, bacterial expression and promoter analysis of squalene synthase from Withania somnifera (L.) Dunal. Gene 2012, 499, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Yamashita, H.; Kajikawa, M.; Ohyama, K.; Nakayachi, O.; Sugiyama, R.; Yamato, K.T.; Muranaka, T.; Fukuzawa, H.; Takemura, M.; et al. Cloning and characterization of a squalene synthase gene from a petroleum plant, Euphorbia tirucalli L. Planta 2009, 229, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Zhao, Y.-J.; Su, P.; Zhang, M.; Tong, Y.-R.; Hu, T.-Y.; Huang, L.-Q.; Gao, W. The MVA pathway genes expressions and accumulation of celastrol in Tripterygium wilfordii suspension cells in response to methyl jasmonate treatment. J. Asian Nat. Prod. Res. 2016, 18, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.S.; Miao, G.P.; Guo, J.; Huo, Y.B.; Zhang, X.; Xie, J.H.; Feng, J.T. Establishment of Tripterygium wilfordii Hook. f. hairy root culture and optimization of its culture conditions for the production of triptolide and wilforine. J. Microbiol. Biotechnol. 2014, 24, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Liu, J.W.; Lu, M.; Ma, Z.Q.; Cai, C.L.; Wang, Y.H.; Zhang, X. Bacterial expression and kinetic analysis of carboxylesterase 001D from Helicoverpa armigera. Int. J. Mol. Sci. 2016, 17, 493. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.P.; Li, W.; Zhang, B.; Zhang, Z.F.; Ma, Z.Q.; Feng, J.T.; Zhang, X.; Zhu, C.S. Identification of genes involved in the biosynthesis of Tripterygium wilfordii Hook. f. secondary metabolites by suppression subtractive hybridization. Plant Mol. Biol. Rep. 2015, 33, 756–769. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Tripterygium wilfordii are available from the authors. |





| Primer Name | Primer Sequence 5′→3′ |
|---|---|
| SQS-F | ATGGGGAGTTTGTGGACGA |
| SQS-R | ACTCGGGGGCTGACATCC |
| SQS-TF | CGGGATCCATGGGGAGTTTGTGGACGA |
| SQS-TR | CGAGCTCATGACTCGCCATTAACTATGCC |
| SQS-QF | GCGCTGCGAGATCCAGCCAT |
| SQS-QR | TGCAGTGAGACCACGCCTCAT |
| EF1α-F | CCAAGGGTGAAAGCAAGGAGAGC |
| EF1α-R | CACTGGTGGTTTTGAGGCTGGTATCT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Liu, Y.; Chen, M.; Feng, J.; Ma, Z.; Zhang, X.; Zhu, C. Cloning, Expression Analysis and Functional Characterization of Squalene Synthase (SQS) from Tripterygium wilfordii. Molecules 2018, 23, 269. https://doi.org/10.3390/molecules23020269
Zhang B, Liu Y, Chen M, Feng J, Ma Z, Zhang X, Zhu C. Cloning, Expression Analysis and Functional Characterization of Squalene Synthase (SQS) from Tripterygium wilfordii. Molecules. 2018; 23(2):269. https://doi.org/10.3390/molecules23020269
Chicago/Turabian StyleZhang, Bin, Yan Liu, Mengmeng Chen, Juntao Feng, Zhiqing Ma, Xing Zhang, and Chuanshu Zhu. 2018. "Cloning, Expression Analysis and Functional Characterization of Squalene Synthase (SQS) from Tripterygium wilfordii" Molecules 23, no. 2: 269. https://doi.org/10.3390/molecules23020269
APA StyleZhang, B., Liu, Y., Chen, M., Feng, J., Ma, Z., Zhang, X., & Zhu, C. (2018). Cloning, Expression Analysis and Functional Characterization of Squalene Synthase (SQS) from Tripterygium wilfordii. Molecules, 23(2), 269. https://doi.org/10.3390/molecules23020269