Pd-Catalyzed, Highly Selective C(sp2)-Br Bond Coupling Reactions of o-(or m-, or p-) Chloromethyl Bromobenzene with Arylboronic Acids
Abstract
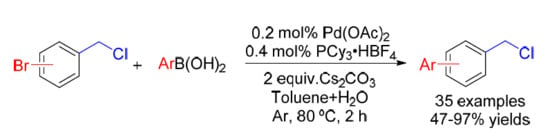
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for the Selective Coupling Reaction of o-(or m-, or p-)chloromethyl Bromobenzene with Arylboronic Acid
3.2. General Procedure for One-Pot Dual Arylations of 1-Bromo-4-(chloromethyl)benzene
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-coupling reactions via organoboranes. J. Organomet. Chem. 2002, 653, 83–90. [Google Scholar] [CrossRef]
- Diedreich, F.; Stang, P.J. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Chowdhury, S.; Georghiou, P. Palladium catalyzed cross-coupling between phenyl- or naphthylboronic acids and benzylic bromides. Tetrahedron Lett. 1999, 40, 7599–7603. [Google Scholar] [CrossRef]
- Oh, C.H.; Lim, Y.M. Double suzuki reactions of organoboronic acids with 1,n-dibromides. Bull. Korean Chem. Soc. 2002, 23, 663–664. [Google Scholar] [CrossRef]
- Lu, T.-Y.; Xue, C.; Luo, F.-T. Palladium-Catalyzed Cross-Coupling Reaction of Aryldioxaborolane with 2-Bromo-N,N-dimethylacetamide. Tetrahedron Lett. 2003, 44, 1587–1590. [Google Scholar] [CrossRef]
- Liu, X.; Deng, M. Remarkable Co-Catalysis by Copper(I) Oxide in the Palladium Catalyzed Cross-Coupling of Arylboronic Acids with Ethyl Bromoacetate. Chem. Commun. 2002, 33, 622–623. [Google Scholar] [CrossRef]
- Gooßen, L. Palladium-Catalyzed Synthesis of Aryl Ketones from Boronic Acids and Carboxylic Acids or Anhydrides. Chem. Commun. 2001, 669–670. [Google Scholar] [CrossRef]
- Netherton, M.R.; Dai, C.; Neuschütz, K.; Fu, G.C. Room-Temperature Alkyl−Alkyl Suzuki Cross-Coupling of Alkyl Bromides that Possess β Hydrogens. J. Am. Chem. Soc. 2001, 123, 10099–10100. [Google Scholar] [CrossRef] [PubMed]
- Langle, S.; Abarbri, M.; Duchêne, A. Selective double Suzuki cross-coupling reactions. Synthesis of unsymmetrical diaryl (or heteroaryl) methanes. Tetrahedron Lett. 2003, 44, 9255–9258. [Google Scholar] [CrossRef]
- Anselmi, E.; Abarbri, M.; Duchêne, A.; Langle-Lamandé, S.; Thibonnet, J. Efficient synthesis of substituted styrenes and biaryls (or heteroaryls) with regioselective reactions of ortho-, meta-, and para-bromobenzyl bromide. Synthesis 2012, 44, 2023–2040. [Google Scholar] [CrossRef]
- Henry, N.; Enguehard-Gueiffier, C.; Thery, I. One-Pot Dual Substitutions of Bromobenzyl Chloride, 2-Chloromethyl-6-halogenoimidazo[1,2-a]pyridine and -[1,2-b]pyridazine by Suzuki–Miyaura Cross-Coupling Reactions. Eur. J. Org. Chem. 2008, 2008, 4824–4827. [Google Scholar] [CrossRef]
- Mahamo, T.; Mogorosi, M.M.; Moss, J.R.; Mapolie, S.F.; Slootweg, J.C.; Lammertsma, K.; Smith, G.S. Neutral palladium (II) complexes with P,N Schiff-base ligands: Synthesis, characterization and application as Suzuki–Miyaura coupling catalysts. J. Organomet. Chem. 2012, 703, 34–42. [Google Scholar] [CrossRef]
- Gurbuz, N.; Özdemir, İ.; Cetinkaya, B.; Seckin, T. Silica-supported 3-4,5-dihydroimidazol-1-yl-propyltriethoxysilanedichloropalladium(II) complex: Heck and Suzuki cross-coupling reactions. Appl. Organomet. Chem. 2003, 17, 776–780. [Google Scholar] [CrossRef]
- Heijnen, D.; Tosi, F.; Vila, C.; Stuart, M.C.; Elsinga, P.H.; Szymanski, W.; Feringa, B.L. Oxygen Activated, Palladium Nanoparticle Catalyzed, Ultrafast Cross-Coupling of Organolithium Reagents. Angew. Chem. Int. Ed. 2017, 129, 3402–3407. [Google Scholar] [CrossRef]
- Gürbüz, N.; Özdemir, I.; Demir, S.; Çetinkaya, B. Improved palladium-catalyzed coupling reactions of aryl halides using saturated N-heterocarbene ligands. J. Mol. Catal. A Chem. 2004, 209, 23–28. [Google Scholar] [CrossRef]
- Mollar, C.; Besora, M.; Maseras, F.; Asensio, G.; Medio-Simón, M. Competitive and Selective Csp3-Br versus Csp2-Br Bond Activation in Palladium-Catalysed Suzuki Cross-Coupling: An Experimental and Theoretical Study of the Role of Phosphine Ligands. Chem. Eur. J. 2010, 16, 13390–13397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, N.; Liu, P.; Ma, X.; Liu, Y.; Xie, J.; Dai, B. Water-soluble salen–Pd complex as an efficient catalyst for Suzuki–Miyaura reaction of sterically hindered substrates in pure water. Tetrahedron 2015, 71, 7985–7989. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, P.; Xie, J.; Dai, B.; Liu, Z. Palladium-catalyzed direct arylation of polyfluoroarene and facile synthesis of liquid crystal compounds. Appl. Organomet. Chem. 2014, 28, 180–185. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Xie, J.; Liu, P.; Dai, B.; He, R. Metallomicelles of palladium(II) complexes as efficient catalysts for the Suzuki–Miyaura reaction in neat water. Appl. Organomet. Chem. 2013, 27, 494–498. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, P.; Xie, J.; Dai, B.; Liu, Z. A rapid and efficient catalysis system for the synthesis of 4-vinylbiphenyl derivatives. Appl. Organomet. Chem. 2013, 27, 707–710. [Google Scholar] [CrossRef]
- Liu, P.; Yan, M.; He, R. Bis(imino) pyridine palladium(II) complexes as efficient catalysts for the Suzuki–Miyaura reaction in water. Appl. Organomet. Chem. 2010, 24, 131–134. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, W.Z.; He, R. Preparation and catalytic properties of bis(imino) pyridine palladium(II) complexes as efficient catalysts for Suzuki cross-coupling reaction in water. Appl. Organomet. Chem. 2009, 23, 135–139. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, L.; Li, X.G.; He, R. Bis(imino) pyridine palladium(II) complexes: Synthesis, structure and catalytic activity. J. Organomet. Chem. 2009, 694, 2290–2294. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Sonawane, H.R.; Hudson, J.C. Sterically Hindered Aromatic Compounds. IV. Solvolysis of t-Butyl-, Phenyl-, and Trialkyl-benzyl Chlorides in Ethanol–Water. Evidence for Steric Acceleration in 2,4,6-Tri-t-butylbenzyl Chloride. Can. J. Chem. 1972, 50, 2318–2325. [Google Scholar] [CrossRef]
- Han, L.; Xia, J.B.; You, L.; Chen, C. Ketone-catalyzed photochemical C(sp3)–H chlorination. Tetrahedron 2017, 73, 3696–3701. [Google Scholar] [CrossRef] [PubMed]
- Burawoy, A.; Spinner, E. Electronic spectra of organic molecules and their interpretation. Part I. Effect of terminal methyl and substituted methyl groups of conjugated systems on K-bands. J. Chem. Soc. 1955, 2557–2563. [Google Scholar] [CrossRef]
- Kauhanka, M.M.; Kauhanka, U.M. Synthesis and properties of some mesogenic benzyl chlorides. Vestsi Nats. Akad. Navuk Belarusi Ser. Khim. Navuk 2010, 64–67. [Google Scholar]
- Filzen, G.F.; Trivedi, B.K.; Geyer, A.G.; Unangst, P.C.; Bratton, L.D. Compounds that Modulate PPAR Activity and Methods for Their Preparation. WO Patent Application No. 2003084916A2, 24 December 2003. [Google Scholar]
- Bartel, S.; Hahn, M.; Moradi, W.A.; Becker, E.; Roelle, T.; Stasch, J.; Schlemmer, K.; Wunder, F.; Knorr, A. Dicarboxylic Acid Derivatives and Their Use. WO Patent Application No. 2007045433 A1, 26 April 2007. [Google Scholar]
- Li, Z.; Liu, C.X.; Xu, X.; Qiu, Q.; Su, X.; Dai, Y.; Yang, J.; Li, H.; Shi, W.; Liao, C.; et al. Discovery of phenylsulfonyl acetic acid derivatives with improved efficacy and safety as potent free fatty acid receptor 1 agonists for the treatment of type 2 diabetes. Eur. J. Med. Chem. 2017, 138, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Hwang, H.S.; Chin, J. Use of the Fetal Reprogramming of a PPAR δ Agonistwo. WO Patent Application No. 2012030165A2, 5 July 2012. [Google Scholar]
- Leivers, M.R.; Roberts, C.D.; Liehr, S.J.R.; Chan, S.A.; Rai, R.; Lauchli, R.; Pham, S.M.; Rajwanshi, V.K.; Ton, T.L. Derivatives of Imidazo [4,5-d] Pyridazine and Their Use as Anti-Viral Compounds. WO Patent Application No. 2009111501 A1, 9 November 2009. [Google Scholar]
- Sabatucci, J.P.; Caufield, C.E.; Greenfield, A.A.; Antane, S. Substituted Naphthylenes for the Treatment of Non-Insulin Dependent Diabetes Mellitus. U.S. Patent Application No. 20130216442 A1, 11 September 2009. [Google Scholar]
- Boettcher, H.; Devant, R.; Greiner, H.; Bartoszyk, G.; Berthelon, J.J.; Noblet, M.; Zeiller, J.J. Preparation of arylamines as central nervous system agents. EP Patent Application No. 707007 A1, 17 April 1996. [Google Scholar]
- Houze, J.; Liu, J.; Ma, Z.H.; Medina, J.C.; Schmitt, M.J.; Sharma, R.; Sun, Y.; Wang, Y.C. Compounds, Pharmaceutical Compositions and Methods for Their Use in Treating Metabolic Disorders. U.S. Patent Application No. 7465804 B2, 16 December 2008. [Google Scholar]
- Standley, E.A.; Jamison, T.F. Simplifying Nickel(0) Catalysis: An Air-Stable Nickel Precatalyst for the Internally Selective Benzylation of Terminal Alkenes. J. Am. Chem. Soc. 2013, 135, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, H.J.; Chang, S. Highly Efficient and Versatile Synthesis of Polyarylfluorenes via Pd-Catalyzed C−H Bond Activation. Org. Lett. 2009, 20, 4588–4591. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, L.M.; Leung, W.P.; Raston, C.L.; Twiss, P.; White, A.H. Axially asymmetric metal alkyls. Part 1. Lithium alkyls of 2,2′-dimethylbiphenyl and its trimethylsilylmethylated compounds: crystal structures of [{Li(Me2NCH2CH2NMe2)}2{(2-CHRC6H4)2}] for R = H (polymeric) and R = SiMe3(monomeric). J. Chem. Soc. Dalton Trans. 1984, 321–329. [Google Scholar]
- Suga, A.; Kazuta, K.; Morihira, K.; Matsuda, K.; Kakuta, H.; Moritani, H.; Iizumi, Y. Novel Benzylamine Derivative. WO Patent Application No. 19959518121 A1, 6 July 1995. [Google Scholar]
- Biediger, R.J.; Bui, H.; Henry, K.M.; Thrash, T. Modulators of c3a Receptor and Methods of Use Thereof. WO Patent Application No. 2008079371 A1, 3 July 2008. [Google Scholar]
- Tao, J.L.; Wang, Z.X. Pincer-Nickel-Catalyzed Cross-Coupling of Aryl Sulfamates with Arylzinc Chlorides. Eur. J. Org. Chem. 2015, 29, 6534–6540. [Google Scholar] [CrossRef]
- Rao, M.L.N.; Dhanorkar, R.J. Pd-catalyzed chemoselective threefold cross-coupling of triarylbismuths with benzylic bromides. RSC Adv. 2013, 3, 6794–6798. [Google Scholar] [CrossRef]
- Futamura, S.; Zong, Z.M. Photobromination of side-chain methyl groups on arenes with N-bromosuccinimide–convenient and selective syntheses of bis(bromomethyl)- and (bromomethyl) methylarenes. Bull. Chem. Soc. Jpn. 1992, 65, 345–348. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, M.T.; Lu, J.M. N-Heterocyclic carbene–palladium(II)–1-methylimidazole complex catalyzed Suzuki–Miyaura coupling of benzylic chlorides with arylboronic acids or potassium phenyltrifluoroborate in neat water. Org. Biomol. Chem. 2013, 11, 2266–2272. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Entry | Pd(OAc)2 | PCy3∙HBF4 | Base/Equiv. | T/°C | Yield/% b |
|---|---|---|---|---|---|
| 1 | 2 mol % | 4 mol % | NEt3 | 80 | (37) c |
| 2 | 2 mol % | 4 mol % | NaOH | 80 | (14) c |
| 3 | 2 mol % | 4 mol % | K3PO4∙3H2O | 80 | (69) c |
| 4 | 2 mol % | 4 mol % | K2CO3 | 80 | (84) c |
| 5 | 2 mol % | 4 mol % | Cs2CO3 | 80 | 99(97) c |
| 6 | 2 mol % | PPh3/4 mol % | Cs2CO3 | 80 | 0 |
| 7 | 2 mol % | 4 mol % | Cs2CO3 | 60 | 74 |
| 8 | 1 mol % | 2 mol % | Cs2CO3 | 80 | 99 |
| 9 | 0.5 mol % | 1 mol % | Cs2CO3 | 80 | 99 |
| 10 | 0.2 mol % | 0.4 mol % | Cs2CO3 | 80 | 99 |
| 11 | 0.1 mol % | 0.2 mol % | Cs2CO3 | 80 | 23 |
 | ||
|---|---|---|
 3b, 79% |  3c, 87% |  3d, 83% |
 3e, 93% |  3f, 83% |  3g, 75% |
 3h, 98% |  3i, 73% |  3j, 90% |
 3k, 47% |  3l, 50% |  3m, 73% |
 3n, 77% |  3o, 86% |  3p, 57% |
 | |||
|---|---|---|---|
 4a, 95% |  4b, 90% |  4c, 92% |  4d, 93% |
 4e, 92% |  4f, 87% |  4g, 95% |  4h, 73% |
 | |||
|---|---|---|---|
 5a, 97% |  5b, 95% |  5c, 89% |  5d, 92% |
 5e, 88% |  5f, 87% |  5g, 95% |  5h, 80% |
 | |
|---|---|
 6a, 78% |  6b, 96% |
 6c, 89% |  6d, 57% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, M.-m.; Liu, P.; Liu, Y.; Lv, X.-m.; Ma, X.-w.; Dai, B. Pd-Catalyzed, Highly Selective C(sp2)-Br Bond Coupling Reactions of o-(or m-, or p-) Chloromethyl Bromobenzene with Arylboronic Acids. Molecules 2018, 23, 433. https://doi.org/10.3390/molecules23020433
Pei M-m, Liu P, Liu Y, Lv X-m, Ma X-w, Dai B. Pd-Catalyzed, Highly Selective C(sp2)-Br Bond Coupling Reactions of o-(or m-, or p-) Chloromethyl Bromobenzene with Arylboronic Acids. Molecules. 2018; 23(2):433. https://doi.org/10.3390/molecules23020433
Chicago/Turabian StylePei, Ming-ming, Ping Liu, Yan Liu, Xin-ming Lv, Xiao-wei Ma, and Bin Dai. 2018. "Pd-Catalyzed, Highly Selective C(sp2)-Br Bond Coupling Reactions of o-(or m-, or p-) Chloromethyl Bromobenzene with Arylboronic Acids" Molecules 23, no. 2: 433. https://doi.org/10.3390/molecules23020433
APA StylePei, M. -m., Liu, P., Liu, Y., Lv, X. -m., Ma, X. -w., & Dai, B. (2018). Pd-Catalyzed, Highly Selective C(sp2)-Br Bond Coupling Reactions of o-(or m-, or p-) Chloromethyl Bromobenzene with Arylboronic Acids. Molecules, 23(2), 433. https://doi.org/10.3390/molecules23020433