Seasonal Reproduction in Vertebrates: Melatonin Synthesis, Binding, and Functionality Using Tinbergen’s Four Questions
Abstract
:1. Introduction
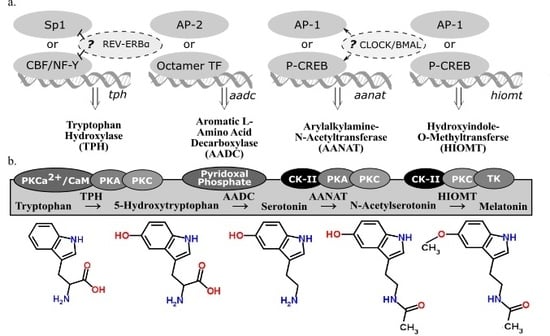
2. Melatonin Synthesis
2.1. Phototransduction Pathways in Mammals and Birds
2.2. Melatonin-Synthesizing Enzymes
2.3. Tryptophan Hydroxylase
2.4. Aromatic l-Amino Acid Decarboxylate
2.5. Arylalkylamine-N-Acetyltransferase
2.6. Hydroxyindole-O-Methyltransferase
2.7. Summary
3. Melatonin Binding
3.1. Melatonin and Autoradiography
3.2. Melatonin Receptor Subtypes
3.3. In Mammals
3.4. In Birds
3.5. In Other Vertebrates
3.6. Summary
4. Melatonin Signalling in Reproduction
4.1. HPG Axis
4.2. In Mammals
4.3. Melatonin in Mammalian Hypothalamus
4.4. Melatonin in Mammalian Pituitary
4.5. Melatonin in Mammalian Gonads
4.6. In Birds
4.7. Melatonin in Avian Hypothalamus
4.8. Melatonin in Avian Pituitary
4.9. Melatonin in Avian Gonads
4.10. Summary
4.11. In Other Vertebrates
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tan, D.-X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.A.; Reiter, R.J. Pineal gland: influence on gonads of male hamsters. Science 1965, 148, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Cassone, V.M. Effects of melatonin on vertebrate circadian systems. Trends Neurosci. 1990, 13, 457–464. [Google Scholar] [CrossRef]
- Klein, D.C.; Moore, R.Y. Pineal N-acetyltransferase and hydroxyindole-O-methyl-transferase: Control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979, 174, 245–262. [Google Scholar] [CrossRef]
- Malpaux, B.; Migaud, M.; Tricoire, H.; Chemineau, P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J. Biol. Rhythm. 2001, 16, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Robert, K.A.; Lesky, J.A.; Partecke, J.; Chambers, B. Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proc. R. Soc. Lond. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D.; Quetting, M.; Partecke, J. Artificial light at night advances avian reproductive physiology. Proc. Biol. Sci. 2013, 280. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.; Ott, L. External and internal factors in sexual activity: Effect of irradiation with different wave-lengths on the mechanisms of photostimulation of the hypophysis and on testicular growth in the immature duck. Yale J. Biol. Med. 1944, 17, 27. [Google Scholar] [PubMed]
- Benoit, J. The role of the eye and of the hypothalamus in the photostimulation of gonads in the duck. Ann. N. Y. Acad. Sci. 1964, 117, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.E. Neither retinal nor pineal photoreceptors mediate photoperiodic control of seasonal reproduction in American tree sparrows (Spizella arborea). J. Exp. Zool. 1991, 259, 117–127. [Google Scholar] [CrossRef]
- Underwood, H.; Siopes, T. Circadian organization in Japanese quali. J. Exp. Zool. 1984, 232, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Thayananuphat, A.; Bakken, T.; El Halawani, M. Dopamine-melatonin neurons in the avian hypothalamus controlling seasonal reproduction. Neuroscience 2007, 150, 223–233. [Google Scholar] [CrossRef] [PubMed]
- El Halawani, M.E.; Kang, S.W.; Leclerc, B.; Kosonsiriluk, S.; Chaiseha, Y. Dopamine–melatonin neurons in the avian hypothalamus and their role as photoperiodic clocks. Gen. Comp. Endocrinol. 2009, 163, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Kuenzel, W.J. Deep-brain photoreceptors (DBPs) involved in the photoperiodic gonadal response in an avian species, Gallus gallus. Gen. Comp. Endocrinol. 2015, 211, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Simonneaux, V.; Ribelayga, C. Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev. 2003, 55, 325–395. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Lee, E.J.; Yun, S.; Choe, H.K.; Park, S.-B.; Son, H.J.; Kim, K.-S.; Dluzen, D.E.; Lee, I.; Hwang, O. Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell 2014, 157, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.L.; Hahn, M.; Kang, U.J.; Joh, T.H. Structure of the Rat Aromatic l-Amino Acid Decarboxylase Gene: Evidence for an Alternative Promoter Usage. J. Neurochem. 1993, 60, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.K.; Nagatsu, T.; Sakurai, T.; Hori, S.; Abe, M.; Matsuda, M. Effect of pyridoxal phosphate deficiency on aromatic l-amino acid decarboxylase activity with L-dopa and l-5-hydroxytryptophan as substrates in rats. Jpn. J. Pharmacol. 1982, 32, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Lovenberg, W.; Jequier, E.; Sjoerdsma, A. Tryptophan hydroxylation: Measurement in pineal gland, brainstem, and carcinoid tumor. Science 1967, 155, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Teerawatanasuk, N.; Carr, L.G. CBF/NF-Y activates transcription of the human tryptophan hydroxylase gene through an inverted CCAAT box. Mol. Brain Res. 1998, 55, 61–70. [Google Scholar] [CrossRef]
- Ly, L.L.; Yoshida, H.; Yamaguchi, M. Nuclear transcription factor Y and its roles in cellular processes related to human disease. Am. J. Cancer Res. 2013, 3, 339–346. [Google Scholar] [PubMed]
- Green, C.B.; Besharse, J.C. Tryptophan hydroxylase expression is regulated by a circadian clock in Xenopus laevis retina. J. Neurochem. 1994, 62, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Juorio, A.; Li, X.-M.; Boulton, A. Aromatic l-amino acid decarboxylase: A neglected and misunderstood enzyme. Neurochem. Res. 1996, 21, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J.; Weissbach, H. Enzymatic O-methylation of N-acetylserotonin to melatonin. Science 1960, 131, 1312. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G. Antioxidant Effects of N-Acetylserotonin. Ann. N. Y. Acad. Sci. 2005, 1053, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-W.; Liu, X.; Pradoldej, S.; Tosini, G.; Chang, Q.; Iuvone, P.M.; Ye, K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. USA 2010, 107, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, N.S.; Borjigin, J.; Snyder, S.H. Rhythmic transcription: the molecular basis of circadian melatonin synthesis. Trends Neurosci. 1997, 20, 487–492. [Google Scholar] [CrossRef]
- Roseboom, P.H.; Namboodiri, M.A.; Zimonjic, D.B.; Popescu, N.C.; Rodriguez, I.R.; Gastel, J.A.; Klein, D.C. Natural melatoninknockdown’in C57BL/6J mice: Rare mechanism truncates serotonin N-acetyltransferase. Mol. Brain Res. 1998, 63, 189–197. [Google Scholar] [CrossRef]
- Klein, D.C. Arylalkylamine N-acetyltransferase: The Timezyme. J. Biol. Chem. 2007, 282, 4233–4237. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.K.; Chik, C.L. Modulation of Aanat gene transcription in the rat pineal gland. J. Neurochem. 2010, 112, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.; Rovsing, L.; Ho, A.K.; Møller, M.; Rath, M.F. Circadian dynamics of the cone-rod homeobox (CRX) transcription factor in the rat pineal gland and its role in regulation of arylalkylamine N-acetyltransferase (AANAT). Endocrinology 2014, 155, 2966–2975. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, P.M.; Tosini, G.; Pozdeyev, N.; Haque, R.; Klein, D.C.; Chaurasia, S.S. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res. 2005, 24, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.; Bashforth, R.; Diack, A.; Andersson, H.; Lincoln, G.; Hazlerigg, D. Rhythmic melatonin secretion does not correlate with the expression of arylalkylamine N-acetyltransferase, inducible cyclic amp early repressor, period1 or cryptochrome1 mRNA in the sheep pineal. Neuroscience 2004, 124, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Akashi, M.; Matsuda, M.; Goto, K.; Miyata, Y.; Node, K.; Nishida, E. Involvement of the protein kinase CK2 in the regulation of mammalian circadian rhythms. Sci. Signal. 2009, 2, ra26. [Google Scholar] [CrossRef] [PubMed]
- Cazaméa-Catalan, D.; Besseau, L.; Falcón, J.; Magnanou, E. The timing of timezyme diversification in vertebrates. PLoS ONE 2014, 9, e112380. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; You, X.; Bian, C.; Yu, H.; Coon, S.L.; Shi, Q. Molecular evolution of aralkylamine N-acetyltransferase in fish: A genomic survey. Int. J. Mol. Sci. 2015, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Guerlotte, J.; Cogne, M.; Greve, P.; Collin, J.; Voisin, P. Transcriptional regulation of hydroxyindole O-methyltransferase in the chicken pineal gland: Day/night changes and long-term effects of light and darkness. Biochem. J. 1993, 290, 661. [Google Scholar] [CrossRef] [PubMed]
- Ribelayga, C.; Gauer, F.; Calgari, C.; Pevet, P.; Simonneaux, V. Photoneural regulation of rat pineal hydroxyindole-O-methyltransferase (HIOMT) messenger ribonucleic acid expression: An analysis of its complex relationship with HIOMT activity. Endocrinology 1999, 140, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Borjigin, J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J. Pineal Res. 2005, 39, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ribelayga, C.; Pévet, P.; Simonneaux, V. HIOMT drives the photoperiodic changes in the amplitude of the melatonin peak of the Siberian hamster. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2000, 278, R1339–R1345. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Dubocovich, M.; Brown, G. Melatonin receptors in peripheral tissues: A new area of melatonin research. Neurosignals 1994, 2, 177–180. [Google Scholar] [CrossRef]
- Stankov, B.; Reiter, R.J. Melatonin receptors: Current status, facts, and hypotheses. Life Sci. 1990, 46, 971–982. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Nowak, J.Z. Characterization of melatonin receptors in the brain of four avian species: Duck, goose, pigeon, and turkey. Gen. Comp. Endocrinol. 1996, 101, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Vernadakis, A.J.; Bemis, W.E.; Bittman, E.L. Localization and partial characterization of melatonin receptors in amphioxus, hagfish, lamprey, and skate. Gen. Comp. Endocrinol. 1998, 110, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.J.; Barrett, P.; Howell, H.E.; Helliwell, R. Melatonin receptors: Localization, molecular pharmacology and physiological significance. Neurochem. Int. 1994, 24, 101–146. [Google Scholar] [CrossRef]
- Weaver, D.R.; Carlson, L.L.; Reppert, S.M. Melatonin receptors and signal transduction in melatonin-sensitive and melatonin-insensitive populations of white-footed mice (Peromyscus leucopus). Brain Res. 1990, 506, 353–357. [Google Scholar] [CrossRef]
- Vaněček, J.; Pavlík, A.; Illnerová, H. Hypothalamic melatonin receptor sites revealed by autoradiography. Brain Res. 1987, 435, 359–362. [Google Scholar] [CrossRef]
- Gauer, F.; Masson-Pévet, M.; Skene, D.J.; Vivien-Roels, B.; Pévet, P. Daily rhythms of melatonin binding sites in the rat pars tuberalis and suprachiasmatic nuclei; evidence for a regulation of melatonin receptors by melatonin itself. Neuroendocrinology 1993, 57, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Morgan, P.; Hastings, M.; Lawson, W.; Davidson, G.; Howell, H. Melatonin receptor sites in the Syrian hamster brain and pituitary. Localization and characterization using [125I] lodomelatonin. J. Neuroendocrinol. 1989, 1, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Maywood, E.S.; Bittman, E.L.; Ebling, F.J.; Barrett, P.; Morgan, P.; Hastings, M.H. Regional distribution of iodomelatonin binding sites within the suprachiasmatic nucleus of the Syrian hamster and the Siberian hamster. J. Neuroendocrinol. 1995, 7, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Masana, M.; Benloucif, S.; Dubocovich, M. Circadian rhythm of mt1 melatonin receptor expression in the suprachiasmatic nucleus of the C3H/HeN mouse1. J. Pineal Res. 2000, 28, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Malpaux, B.; Daveau, A.; Maurice-Mandon, F.; Duarte, G.; Chemineau, P. Evidence That Melatonin Acts in the Premammillary Hypothalamic Area to Control Reproduction in the Ewe: Presence of Binding Sites and Stimulation of Luteinizing Hormone Secretion by in Situ Microimplant Delivery 1. Endocrinology 1998, 139, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.; Ball, G. Photoperiod-dependent and-independent regulation of melatonin receptors in the forebrain of songbirds. J. Neuroendocrinol. 2000, 12, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.E.; Van’t Hof, T.J.; Ball, G.F. Seasonal neuroplasticity in the songbird telencephalon: A role for melatonin. Proc. Natl. Acad. Sci. USA 1999, 96, 4674–4679. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.E.; Perfito, N.; Calisi, R.M. Season-and context-dependent sex differences in melatonin receptor activity in a forebrain song control nucleus. Horm. Behav. 2013, 63, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Calisi, R.M.; Bentley, G.E. Lab and field experiments: Are they the same animal? Horm. Behav. 2009, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gahr, M.; Kosar, E. Identification, distribution, and developmental changes of a melatonin binding site in the song control system of the zebra finch. J. Comp. Neurol. 1996, 367, 308–318. [Google Scholar] [CrossRef]
- Whitfield-Rucker, M.G.; Cassone, V.M. Melatonin binding in the house sparrow song control system: Sexual dimorphism and the effect of photoperiod. Horm. Behav. 1996, 30, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Aste, N.; Cozzi, B.; Stankov, B.; Panzica, G. Sexual differences and effect of photoperiod on melatonin receptor in avian brain. Microsc. Res. Tech. 2001, 55, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Cassone, V.; Brooks, D.; Kelm, T. Comparative distribution of 2 [125I] iodomelatonin binding in the brains of diurnal birds: Outgroup analysis with turtles. Brain Behav. Evol. 1995, 45, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Gene regulation by melatonin. Ann. N. Y. Acad. Sci. 2000, 917, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef] [PubMed]
- Nosjean, O.; Nicolas, J.P.; Klupsch, F.; Delagrange, P.; Canet, E.; Boutin, J.A. Comparative pharmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2. Tissue distribution of MT3/QR2. Biochem. Pharmacol. 2001, 61, 1369–1379. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Witt-Enderby, P.A.; Bennett, J.; Jarzynka, M.J.; Firestine, S.; Melan, M.A. Melatonin receptors and their regulation: Biochemical and structural mechanisms. Life Sci. 2003, 72, 2183–2198. [Google Scholar] [CrossRef]
- Morgan, P.J.; Hazlerigg, D.G. Photoperiodic signalling through the melatonin receptor turns full circle. J. Neuroendocrinol. 2008, 20, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Smith, D.G.; Hardeland, R.; Yang, M.Y.; Xu, H.L.; Zhang, L.; Yin, H.D.; Zhu, Q. Melatonin receptor genes in vertebrates. Int. J. Mol. Sci. 2013, 14, 11208–11223. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Audic, S.; Claverie, J.-M.; Blanc, G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol. Biol. 2010, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [PubMed]
- Naji, L.; Carrillo-Vico, A.; Guerrero, J.M.; Calvo, J.R. Expression of membrane and nuclear melatonin receptors in mouse peripheral organs. Life Sci. 2004, 74, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Pang, S. Melatonin and its receptors in the gastrointestinal tract. Neurosignals 1994, 2, 181–193. [Google Scholar] [CrossRef]
- Natesan, A.K.; Cassone, V.M. Melatonin receptor mRNA localization and rhythmicity in the retina of the domestic chick, Gallus domesticus. Vis. Neurosci. 2002, 19, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Helfer, G.; Brandstätter, R. Melatonin receptor expression in the zebra finch brain and peripheral tissues. Chronobiol. Int. 2012, 29, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Dong, Y.; Cao, J.; Wang, Z.; Zhang, Z.; Chen, Y. Developmental changes of melatonin receptor expression in the spleen of the chicken, Gallus domesticus. Acta Histochem. 2015, 117, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Pévet, P. Melatonin receptors as therapeutic targets in the suprachiasmatic nucleus. Expert Opin. Ther. Targets 2016, 20, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, S.; Yoshimura, T.; Ebihara, S.; Korf, H.-W. Melatonin transmits photoperiodic signals through the MT1 melatonin receptor. J. Neurosci. 2009, 29, 2885–2889. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.R.; Liu, C.; Reppert, S.M. Nature’s knockout: The Mel1b receptor is not necessary for reproductive and circadian responses to melatonin in Siberian hamsters. Mol. Endocrinol. 1996, 10, 1478–1487. [Google Scholar] [PubMed]
- Prendergast, B.J. MT1 melatonin receptors mediate somatic, behavioral, and reproductive neuroendocrine responses to photoperiod and melatonin in Siberian hamsters (Phodopus sungorus). Endocrinology 2010, 151, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Gauer, F.; Schuster, C.; Poirel, V.-J.; Pévet, P.; Masson-Pévet, M. Cloning experiments and developmental expression of both melatonin receptor Mel 1A mRNA and melatonin binding sites in the Syrian hamster suprachiasmatic nuclei. Mol. Brain Res. 1998, 60, 193–202. [Google Scholar] [CrossRef]
- Dubocovich, M.L. Melatonin receptors: Are there multiple subtypes? Trends Pharmacol. Sci. 1995, 16, 50–56. [Google Scholar] [CrossRef]
- Ebisawa, T.; Karne, S.; Lerner, M.R.; Reppert, S.M. Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc. Natl. Acad. Sci. USA 1994, 91, 6133–6137. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R.; Ebisawa, T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994, 13, 1177–1185. [Google Scholar] [CrossRef]
- Molinari, E.J.; North, P.C.; Dubocovich, M.L. 2-[125I] iodo-5-methoxycarbonylamino-N-acetyltryptamine: A selective radioligand for the characterization of melatonin ML 2 binding sites. Eur. J. Pharmacol. 1996, 301, 159–168. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Yun, K.; Al-ghoul, W.M.; Benloucif, S.; Masana, M.I. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998, 12, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-X.; Zhu, C.-B.; Xu, S.-F.; Cao, X.-D.; Wu, G.-C. Selective MT 2 melatonin receptor antagonist blocks melatonin-induced antinociception in rats. Neurosci. Lett. 2000, 282, 161–164. [Google Scholar] [CrossRef]
- Hunt, A.E.; Al-Ghoul, W.M.; Gillette, M.U.; Dubocovich, M.L. Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am. J. Physiol. Cell Physiol. 2001, 280, C110–C118. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Mogilnicka, E.; Areso, P.M. Antidepressant-like activity of the melatonin receptor antagonist, luzindole (N-0774), in the mouse behavioral despair test. Eur. J. Pharmacol. 1990, 182, 313–325. [Google Scholar] [CrossRef]
- Sallinen, P.; Saarela, S.; Ilves, M.; Vakkuri, O.; Leppäluoto, J. The expression of MT1 and MT2 melatonin receptor mRNA in several rat tissues. Life Sci. 2005, 76, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Weaver, D.R.; Jin, X.; Shearman, L.P.; Pieschl, R.L.; Gribkoff, V.K.; Reppert, S.M. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 1997, 19, 91–102. [Google Scholar] [CrossRef]
- Sumaya, I.; Masana, M.; Dubocovich, M. The antidepressant-like effect of the melatonin receptor ligand luzindole in mice during forced swimming requires expression of MT2 but not MT1 melatonin receptors. J. Pineal Res. 2005, 39, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Adamah-Biassi, E.; Hudson, R.; Dubocovich, M. Genetic deletion of MT1 melatonin receptors alters spontaneous behavioral rhythms in male and female C57BL/6 mice. Horm. Behav. 2014, 66, 619–627. [Google Scholar] [CrossRef] [PubMed]
- von Gall, C.; Stehle, J.H.; Weaver, D.R. Mammalian melatonin receptors: Molecular biology and signal transduction. Cell Tissue Res. 2002, 309, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Owino, S.; Guillaume, J.; Jockers, R. Understanding melatonin receptor pharmacology: Latest insights from mouse models, and their relevance to human disease. Bioessays 2014, 36, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Petterborg, L.; Richardson, B.; Reiter, R. Effect of long or short photoperiod on pineal melatonin content in the white-footed mouse, Peromyscus leucopus. Life Sci. 1981, 29, 1623–1627. [Google Scholar] [CrossRef]
- Lynch, G.R.; Heath, H.W.; Johnston, C.M. Effect of geographical origin on the photoperiodic control of reproduction in the white-footed mouse, Peromyscus leucopus. Biol. Reprod. 1981, 25, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Heath, H.W.; Lynch, G.R. Intraspecific differences for melatonin-induced reproductive regression and the seasonal molt in Peromyscus leucopus. Gen. Comp. Endocrinol. 1982, 48, 289–295. [Google Scholar] [CrossRef]
- Heideman, P.D.; Kane, S.L.; Goodnight, A.L. Differences in hypothalamic 2-[125I] iodomelatonin binding in photoresponsive and non-photoresponsive white-footed mice, Peromyscus leucopus. Brain Res. 1999, 840, 56–64. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Herring, K.L.; Walton, J.C.; Fonken, L.K.; Weil, Z.M.; Nelson, R.J. Evidence for feedback control of pineal melatonin secretion. Neurosci. Lett. 2013, 542, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Gerdin, M.J.; Masana, M.I.; Dubocovich, M.L. Melatonin-mediated regulation of human MT 1 melatonin receptors expressed in mammalian cells. Biochem. Pharmacol. 2004, 67, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Tamura, H.; Reiter, R.J. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: Hypothesis and significance. J. Pineal Res. 2007, 43, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Boussard, M.-F.; Truche, S.; Rousseau-Rojas, A.; Briss, S.; Descamps, S.; Droual, M.; Wierzbicki, M.; Ferry, G.; Audinot, V.; Delagrange, P. New ligands at the melatonin binding site MT3. Eur. J. Med. Chem. 2006, 41, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Nosjean, O.; Ferro, M.; Cogé, F.; Beauverger, P.; Henlin, J.-M.; Lefoulon, F.; Fauchère, J.-L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the Melatonin-binding Site MT3 as the Quinone Reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef] [PubMed]
- Pintor, J.; Peláez, T.; Hoyle, C.H.; Peral, A. Ocular hypotensive effects of melatonin receptor agonists in the rabbit: Further evidence for an MT3 receptor. Br. J. Pharmacol. 2003, 138, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Serle, J.B.; Wang, R.-F.; Peterson, W.M.; Plourde, R.; Yerxa, B.R. Effect of 5-MCA-NAT, a putative melatonin MT3 receptor agonist, on intraocular pressure in glaucomatous monkey eyes. J. Glaucoma 2004, 13, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Marcheteau, E.; Hennig, P.; Moulharat, N.; Berger, S.; Delagrange, P.; Bouchet, J.; Ferry, G. MT3/QR2 melatonin binding site does not use melatonin as a substrate or a co-substrate. J. Pineal Res. 2008, 45, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Vincent, L.; Cohen, W.; Delagrange, P.; Boutin, J.A.; Nosjean, O. Molecular and cellular pharmacological properties of 5-methoxycarbonylamino-N-acetyltryptamine (MCA-NAT): A nonspecific MT3 ligand. J. Pineal Res. 2010, 48, 222–229. [Google Scholar] [CrossRef] [PubMed]
- De Sampaio, L.F.S. An unexpected effect of 5-MCA-NAT in chick retinal development. Int. J. Dev. Neurosci. 2009, 27, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, R.V.; Conceição, D.S.B.; Miranda, M.S.; Lucia de Fatima, S.S.; Ohashi, O.M. MT3 melatonin binding site, MT1 and MT2 melatonin receptors are present in oocyte, but only MT1 is present in bovine blastocyst produced in vitro. Reprod. Biol. Endocrinol. 2012, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.-X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Masana, M.I.; Erşahin, Ç.; Dubocovich, M.L. Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J. Pharmacol. Exp. Ther. 2003, 306, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Gerdin, M.J.; Masana, M.I.; Rivera-Bermudez, M.A.; Hudson, R.L.; Earnest, D.J.; Gillette, M.U.; Dubocovich, M.L. Melatonin desensitizes endogenous MT2 melatonin receptors in the rat suprachiasmatic nucleus: Relevance for defining the periods of sensitivity of the mammalian circadian clock to melatonin. FASEB J. 2004, 18, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Bondi, C.D.; McKeon, R.M.; Bennett, J.M.; Ignatius, P.F.; Brydon, L.; Jockers, R.; Melan, M.A.; Witt-Enderby, P.A. MT1 melatonin receptor internalization underlies melatonin-induced morphologic changes in Chinese hamster ovary cells and these processes are dependent on Gi proteins, MEK 1/2 and microtubule modulation. J. Pineal Res. 2008, 44, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Ayre, E.; Pang, S. 2-[125I] iodomelatonin binding sites in the testis and ovary: Putative melatonin receptors in the gonads. Neurosignals 1994, 3, 71–84. [Google Scholar] [CrossRef]
- González-Arto, M.; Vicente-Carrillo, A.; Martínez-Pastor, F.; Fernández-Alegre, E.; Roca, J.; Miró, J.; Rigau, T.; Rodríguez-Gil, J.E.; Pérez-Pé, R.; Muiño-Blanco, T. Melatonin receptors MT 1 and MT 2 are expressed in spermatozoa from several seasonal and nonseasonal breeder species. Theriogenology 2016, 86, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Izzo, G.; Francesco, A.; Ferrara, D.; Campitiello, M.R.; Serino, I.; Minucci, S.; d’Istria, M. Expression of melatonin (MT1, MT2) and melatonin-related receptors in the adult rat testes and during development. Zygote 2010, 18, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L. Inhibition of rat testicular androgen synthesis in vitro by melantonin and serotonin. Endocrinology 1972, 90, 17. [Google Scholar] [PubMed]
- Tijmes, M.; Pedraza, R.; Valladares, L. Melatonin in the rat testis: Evidence for local synthesis. Steroids 1996, 61, 65–68. [Google Scholar] [CrossRef]
- Stefulj, J.; Hörtner, M.; Ghosh, M.; Schauenstein, K.; Rinner, I.; Wölfler, A.; Semmler, J.; Liebmann, P.M. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 2001, 30, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Rivkees, S.A.; Cassone, V.M.; Weaver, D.R.; Reppert, S.M. Melatonin Receptors in Chick Brain: Characterization and Localization. Endocrinology 1989, 125, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, B.; Stankov, B.; Viglietti-Panzica, C.; Capsoni, S.; Aste, N.; Lucini, V.; Fraschini, F.; Panzica, G. Distribution and characterization of melatonin receptors in the brain of the Japanese quail, Coturnix japonica. Neurosci. Lett. 1993, 150, 149–152. [Google Scholar] [CrossRef]
- Panzica, G.; Fraschini, F.; Aste, N.; Lucini, V.; Viglietti-Panzica, C.; Cozzi, B.; Stankov, B. The density of melatonin receptors is dependent upon the prevailing photoperiod in the Japanese quail (Coturnix japonica). Neurosci. Lett. 1994, 173, 111–114. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin receptor subtypes Mel1a and Mel1c but not Mel1b are associated with monochromatic light-induced B-lymphocyte proliferation in broilers. Domest. Anim. Endocrinol. 2013, 45, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R.; Cassone, V.M.; Godson, C.; Kolakowski, L.F. Melatonin receptors are for the birds: Molecular analysis of two receptor subtypes differentially expressed in chick brain. Neuron 1995, 15, 1003–1015. [Google Scholar] [CrossRef]
- Ubuka, T.; Bentley, G.E.; Ukena, K.; Wingfield, J.C.; Tsutsui, K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Natl. Acad. Sci. USA 2005, 102, 3052–3057. [Google Scholar] [CrossRef] [PubMed]
- Fusani, L.; Gahr, M. Differential expression of melatonin receptor subtypes MelIa, MelIb and MelIc in relation to melatonin binding in the male songbird brain. Brain Behav. Evol. 2014, 85, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Metzdorf, R.; van der Roest, M.; Fusani, L.; ter Maat, A.; Gahr, M. Melatonin affects the temporal organization of the song of the zebra finch. FASEB J. 2005, 19, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.; Leo, M.M.; Subramani, J.; Anish, D.; Sudhagar, M.; Ahmed, K.; Saxena, M.; Tyagi, J.; Sastry, K.; Saxena, V. Expression analysis of melatonin receptor subtypes in the ovary of domestic chicken. Vet. Res. Commun. 2009, 33, 49–56. [Google Scholar] [CrossRef] [PubMed]
- McGuire, N.L.; Kangas, K.; Bentley, G.E. Effects of melatonin on peripheral reproductive function: Regulation of testicular GnIH and testosterone. Endocrinology 2011, 152, 3461–3470. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Haldar, C. Reciprocal interaction between melatonin receptors (Mel 1a, Mel 1b, and Mel 1c) and androgen receptor (AR) expression in immunoregulation of a seasonally breeding bird, Perdicula asiatica: Role of photoperiod. J. Photochem. Photobiol. B Biol. 2013, 122, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, N.; Tu, J.; Hu, Y.; Yang, M.; Yin, H.; Chen, B.; Xu, H.; Yao, Y.; Zhu, Q. Expression patterns of melatonin receptors in chicken ovarian follicles affected by monochromatic light. Genet. Mol. Res. 2015, 14, 10072–10080. [Google Scholar] [CrossRef] [PubMed]
- Csernus, V.; Becher, P.; Mess, B. Wavelength dependency of light-induced changes in rhythmic melatonin secretion from chicken pineal gland in vitro. Neuro Endocrinol. Lett. 1998, 20, 299–304. [Google Scholar]
- Torii, M.; Kojima, D.; Okano, T.; Nakamura, A.; Terakita, A.; Shichida, Y.; Wada, A.; Fukada, Y. Two isoforms of chicken melanopsins show blue light sensitivity. FEBS Lett. 2007, 581, 5327–5331. [Google Scholar] [CrossRef] [PubMed]
- Burstein, D.; Harrington, L.B.; Strutt, S.C.; Probst, A.J.; Anantharaman, K.; Thomas, B.C.; Doudna, J.A.; Banfield, J.F. New CRISPR–Cas systems from uncultivated microbes. Nature 2017, 542, 237. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- McCord, C.P.; Allen, F.P. Evidences associating pineal gland function with alterations in pigmentation. J. Exp. Zool. 1917, 23, 207–224. [Google Scholar] [CrossRef]
- Joss, J.M. The pineal complex, melatonin, and color change in the lamprey Lampetra. Gen. Comp. Endocrinol. 1973, 21, 188–195. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Delgado, M.J.; Gutiérrez, P.; Alonso-Bedate, M. Melatonin and photoperiod alter growth and larval development in Xenopus laevis tadpoles. Comp. Biochem. Physiol. Part A Physiol. 1987, 86, 417–421. [Google Scholar] [CrossRef]
- Bolliet, V.; Ali, M.; Anctil, M.; Zachmann, A. Melatonin secretion in vitro from the pineal complex of the lamprey Petromyzon marinus. Gen. Comp. Endocrinol. 1993, 89, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Samejima, M.; Tamotsu, S.; Uchida, K.; Moriguchi, Y.; Morita, Y. Melatonin excretion rhythms in the cultured pineal organ of the lamprey, Lampetra japonica. Biol. Signal. 1997, 6, 241–246. [Google Scholar]
- Larson-Prior, L.J.; Siuciak, J.A.; Dubocovich, M.L. Localization of 2-[125I] iodomelatonin binding sites in visual areas of the turtle brain. Eur. J. Pharmacol. 1996, 297, 181–185. [Google Scholar] [CrossRef]
- Sauzet, S.; Besseau, L.; Perez, P.H.; Covès, D.; Chatain, B.; Peyric, E.; Boeuf, G.; Muñoz-Cueto, J.A.; Falcón, J. Cloning and retinal expression of melatonin receptors in the European sea bass, Dicentrarchus labrax. Gen. Comp. Endocrinol. 2008, 157, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Confente, F.; Rendón, M.C.; Besseau, L.; Falcón, J.; Muñoz-Cueto, J.A. Melatonin receptors in a pleuronectiform species, Solea senegalensis: Cloning, tissue expression, day–night and seasonal variations. Gen. Comp. Endocrinol. 2010, 167, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.Y.; Hong, W.S.; Zhu, W.B.; Shi, Q.; You, X.X.; Chen, S.X. Cloning and expression of melatonin receptors in the mudskipper boleophthalmus pectinirostris: Their role in synchronizing its semilunar spawning rhythm. Gen. Comp. Endocrinol. 2014, 195, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Claes, J.M.; Mallefet, J. Hormonal control of luminescence from lantern shark (Etmopterus spinax) photophores. J. Exp. Biol. 2009, 212, 3684–3692. [Google Scholar] [CrossRef] [PubMed]
- Filadelfi, A.M.C.; de Castrucci, A.M.L. Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates. J. Pineal Res. 1996, 20, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [Green Version]
- Chai, K.; Liu, X.; Zhang, Y.; Lin, H. Day-night and reproductive cycle profiles of melatonin receptor, kiss, and gnrh expression in orange-spotted grouper (Epinephelus coioides). Mol. Reprod. Dev. 2013, 80, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Park, J.-G.; Takeuchi, Y.; Hur, S.-P.; Lee, Y.-D.; Kim, S.-J.; Takemura, A. Influence of moonlight on mRNA expression patterns of melatonin receptor subtypes in the pineal organ of a tropical fish. Mar. Genom. 2014, 14, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, T.; Takeuchi, Y.; Hur, S.-P.; Takemura, A. Impacts of moonlight on fish reproduction. Mar. Genom. 2014, 14, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Takemura, A.; Ueda, S.; Hiyakawa, N.; Nikaido, Y. A direct influence of moonlight intensity on changes in melatonin production by cultured pineal glands of the golden rabbitfish, Siganus guttatus. J. Pineal Res. 2006, 40, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Brainard, G.C.; Richardson, B.A.; Hurlbut, E.C.; Steinlechner, S.; Matthews, S.A.; Reiter, R.J. The influence of various irradiances of artificial light, twilight, and moonlight on the suppression of pineal melatonin content in the Syrian hamster. J. Pineal Res. 1984, 1, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Nürnberger, F.; Joshi, B.N.; Heinzeller, T.; Milin, J.; Reiter, R.J. Responsiveness of Pineal N-Acetyltransferase and Melatonin in the Cotton Rat Exposed to Either Artificial or Natural Light at Night. J. Pineal Res. 1985, 2, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Schoech, S.J.; Bowman, R.; Hahn, T.P.; Goymann, W.; Schwabl, I.; Bridge, E.S. The effects of low levels of light at night upon the endocrine physiology of western scrub-jays (Aphelocoma californica). J. Exp. Zool. Part A Ecol. Genet. Physiol. 2013, 319, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Tinbergen, N. On aims and methods of ethology. Ethology 1963, 20, 410–433. [Google Scholar] [CrossRef]
- Sandow, J. The regulation of LHRH action at the pituitary and gonadal receptor level: A review. Psychoneuroendocrinology 1983, 8, 277–297. [Google Scholar] [CrossRef]
- Millar, R.; Lowe, S.; Conklin, D.; Pawson, A.; Maudsley, S.; Troskie, B.; Ott, T.; Millar, M.; Lincoln, G.; Sellar, R. A novel mammalian receptor for the evolutionarily conserved type II GnRH. Proc. Natl. Acad. Sci. USA 2001, 98, 9636–9641. [Google Scholar] [CrossRef] [PubMed]
- Pawson, A.J.; Morgan, K.; Maudsley, S.R.; Millar, R.P. Type II gonadotrophin-releasing hormone (GnRH-II) in reproductive biology. Reproduction 2003, 126, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Roch, G.J.; Busby, E.R.; Sherwood, N.M. GnRH receptors and peptides: Skating backward. Gen. Comp. Endocrinol. 2014, 209, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Revel, F.G.; Ansel, L.; Klosen, P.; Saboureau, M.; Pévet, P.; Mikkelsen, J.D.; Simonneaux, V. Kisspeptin: A key link to seasonal breeding. Rev. Endocr. Metab. Disord. 2007, 8, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Greives, T.J.; Mason, A.O.; Scotti, M.-A.L.; Levine, J.; Ketterson, E.D.; Kriegsfeld, L.J.; Demas, G.E. Environmental control of kisspeptin: Implications for seasonal reproduction. Endocrinology 2007, 148, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.E.; Kriegsfeld, L.J.; Osugi, T.; Ukena, K.; O’brien, S.; Perfito, N.; Moore, I.T.; Tsutsui, K.; Wingfield, J.C. Interactions of gonadotropin-releasing hormone (GnRH) and gonadotropin-inhibitory hormone (GnIH) in birds and mammals. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2006, 305, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Bentley, G.E.; Bedecarrats, G.; Osugi, T.; Ubuka, T.; Kriegsfeld, L.J. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front. Neuroendocrinol. 2010, 31, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Kriegsfeld, L.J.; Ubuka, T.; Bentley, G.E.; Tsutsui, K. Seasonal control of gonadotropin-inhibitory hormone (GnIH) in birds and mammals. Front. Neuroendocrinol. 2015, 37, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, V.; Karsch, F.; Lee, J. Hypothalamic, pituitary and gonadal regulation of FSH. Reprod. Suppl. 2001, 59, 67–82. [Google Scholar]
- Thompson, I.R.; Kaiser, U.B. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol. Cell. Endocrinol. 2014, 385, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.-Y. Inhibins, activins, and follistatins: Gonadal proteins modulating the secretion of follicle-stimulating hormone. Endoc. Rev. 1988, 9, 267–293. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Ukena, K.; Sharp, P.J.; Bentley, G.E.; Tsutsui, K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology 2006, 147, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Smitz, J. Luteinizing hormone and human chorionic gonadotropin: Origins of difference. Mol. Cell. Endocrinol. 2014, 383, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H.; Cheng, J. FSH and testosterone signaling in Sertoli cells. Reproduction 2005, 130, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Chappel, S.C.; Howles, C. Review Reevaluation of the roles of luteinizing hormone and follicle-stimulating hormone in the ovulatory process. Hum. Reprod. 1991, 6, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Karsch, F.J. Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Ann. Rev. Physiol. 1987, 49, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Lavoie, H.B.; Bo-Abbas, Y.; Hall, J.E. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- McGuire, N.L.; Bentley, G.E. Neuropeptides in the gonads: From evolution to pharmacology. Front. Pharmacol. 2010, 1, 114. [Google Scholar] [CrossRef] [PubMed]
- Carreau, S.; Genissel, C.; Bilinska, B.; Levallet, J. Topical review: Sources of oestrogen in the testis and reproductive tract of the male. Int. J. Androl. 1999, 22, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.; Carnes, K. The role of estrogen in testis and the male reproductive tract: A review and species comparison. Anim. Reprod. 2004, 1, 5–30. [Google Scholar]
- Hess, R.A. Estrogen in the adult male reproductive tract: A review. Reprod. Biol. Endocrinol. 2003, 1, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruska, K.P.; Fernald, R.D. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology 2011, 26, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Kaiser, F.E.; Perry, H.M.; Patrick, P.; Morley, P.M.; Stauber, P.M.; Vellas, B.; Baumgartner, R.N.; Garry, P.J. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 1997, 46, 410–413. [Google Scholar] [CrossRef]
- Peper, J.S.; Brouwer, R.M.; van Leeuwen, M.; Schnack, H.G.; Boomsma, D.I.; Kahn, R.S.; Pol, H.E.H. HPG-axis hormones during puberty: A study on the association with hypothalamic and pituitary volumes. Psychoneuroendocrinology 2010, 35, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Revel, F.G.; Masson-Pévet, M.; Pévet, P.; Mikkelsen, J.D.; Simonneaux, V. Melatonin controls seasonal breeding by a network of hypothalamic targets. Neuroendocrinology 2009, 90, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Smoot, R.; Weller, J.; Higa, S.; Markey, S.; Creed, G.; Jacobowitz, D. Lesions of the paraventricular nucleus area of the hypothalamus disrupt the suprachiasmatic → spinal cord circuit in the melatonin rhythm generating system. Brain Res. Bull. 1983, 10, 647–652. [Google Scholar] [CrossRef]
- Bittman, E.; Crandell, R.; Lehman, M. Influences of the paraventricular and suprachiasmatic nuclei and olfactory bulbs on melatonin responses in the golden hamster. Biol. Reprod. 1989, 40, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.; Roberts, A.; Herbert, J. Neurotoxic lesions of the anterior hypothalamus disrupt the photoperiodic but not the circadian system of the Syrian hamster. Neuroendocrinology 1985, 40, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.; Walker, A.; Roberts, A.; Herbert, J. Intra-hypothalamic melatonin blocks photoperiodic responsiveness in the male Syrian hamster. Neuroscience 1988, 24, 987–991. [Google Scholar] [CrossRef]
- Gingerich, S.; Wang, X.; Lee, P.; Dhillon, S.; Chalmers, J.; Koletar, M.; Belsham, D. The generation of an array of clonal, immortalized cell models from the rat hypothalamus: Analysis of melatonin effects on kisspeptin and gonadotropin-inhibitory hormone neurons. Neuroscience 2009, 162, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Ansel, L.; Bentsen, A.H.; Ancel, C.; Bolborea, M.; Klosen, P.; Mikkelsen, J.D.; Simonneaux, V. Peripheral kisspeptin reverses short photoperiod-induced gonadal regression in Syrian hamsters by promoting GNRH release. Reproduction 2011, 142, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Simonneaux, V.; Ansel, L.; Revel, F.G.; Klosen, P.; Pévet, P.; Mikkelsen, J.D. Kisspeptin and the seasonal control of reproduction in hamsters. Peptides 2009, 30, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Angelini, N.L.; Fujieda, H.; Brown, G.M.; Belsham, D.D. Cyclical regulation of GnRH gene expression in GT1–7 GnRH-secreting neurons by melatonin. Endocrinology 2001, 142, 4711–4720. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Belsham, D.D. Melatonin Receptor Activation Regulates GnRH Gene Expression and Secretion in GT1–7 GnRH Neurons Signal Transduction Mechanisms. J. Biol. Chem. 2002, 277, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Maywood, E.S.; Bittman, E.L.; Hastings, M.H. Lesions of the melatonin-and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol. Reprod. 1996, 54, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Jarjisian, S.G.; Piekarski, D.J.; Place, N.J.; Driscoll, J.R.; Paxton, E.G.; Kriegsfeld, L.J.; Zucker, I. Dorsomedial hypothalamic lesions block Syrian hamster testicular regression in short day lengths without diminishing increased testosterone negative-feedback sensitivity. Biol. Reprod. 2013, 89, 23. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.O.; Duffy, S.; Zhao, S.; Ubuka, T.; Bentley, G.E.; Tsutsui, K.; Silver, R.; Kriegsfeld, L.J. Photoperiod and reproductive condition are associated with changes in RFamide-related peptide (RFRP) expression in Syrian hamsters (Mesocricetus auratus). J. Biol. Rhythm. 2010, 25, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ancel, C.; Bentsen, A.H.; Sébert, M.-E.; Tena-Sempere, M.; Mikkelsen, J.D.; Simonneaux, V. Stimulatory Effect of RFRP-3 on the Gonadotrophic Axis in the Male Syrian Hamster: The Exception Proves the Rule. Endocrinology 2012, 153, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Simonneaux, V.; Ancel, C.; Gauer, F.; Poirel, V.-J. Kisspeptins and RFRP-3 act in concert to synchronize rodent reproduction with seasons. Front. Neurosci. 2013, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, J.B.; Poirel, V.; Mikkelsen, J.D.; Tsutsui, K.; Simonneaux, V.; Gauer, F. Sex differences in the photoperiodic regulation of RF-Amide related peptide (RFRP) and its receptor GPR147 in the Syrian hamster. J. Comp. Neurol. 2015, 524, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
- Petri, I.; Diedrich, V.; Wilson, D.; Fernández-Calleja, J.; Herwig, A.; Steinlechner, S.; Barrett, P. Orchestration of gene expression across the seasons: Hypothalamic gene expression in natural photoperiod throughout the year in the Siberian hamster. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Malpaux, B.; Viguie, C.; Skinner, D.; Thiery, J.; Pelletier, J.; Chemineau, P. Seasonal breeding in sheep: Mechanism of action of melatonin. Anim. Reprod. Sci. 1996, 42, 109–117. [Google Scholar] [CrossRef]
- Li, Q.; Rao, A.; Pereira, A.; Clarke, I.; Smith, J. Kisspeptin cells in the ovine arcuate nucleus express prolactin receptor but not melatonin receptor. J. Neuroendocrinol. 2011, 23, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Posbergh, C.; Murphy, R.; Thonney, M. Further testing of Melatonin Receptor 1a for out-of-season reproduction in the Cornell flock and allelic frequencies compared with Romney sheep. J. Anim. Sci. 2017, 95, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T. Kisspeptin signalling in the brain: Steroid regulation in the rodent and ewe. Brain Res. Rev. 2008, 57, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Birnie, M.; Lincoln, G.; Hazlerigg, D. RFamide-Related Peptide and its Cognate Receptor in the Sheep: cDNA Cloning, mRNA Distribution in the Hypothalamus and the Effect of Photoperiod. J. Neuroendocrinol. 2008, 20, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Knobil, E. The wisdom of the body revisited. Physiology 1999, 14, 1–11. [Google Scholar] [CrossRef]
- Christian, C.A.; Moenter, S.M. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr. Rev. 2010, 31, 544–577. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Hannah, L.T.; Hastings, M.H.; Maywood, E.S. Melatonin receptors in the rat brain and pituitary. J. Pineal Res. 1995, 19, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.; Stehle, J.; Stopa, E.; Reppert, S. Melatonin receptors in human hypothalamus and pituitary: Implications for circadian and reproductive responses to melatonin. J. Clin. Endocrinol. Metab. 1993, 76, 295–301. [Google Scholar] [PubMed]
- Martin, J.; Sattler, C. Selectivity of melatonin pituitary inhibition for luteinizing hormone-releasing hormone. Neuroendocrinology 1982, 34, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Vaněček, J.; Janský, L. Short days induce changes in specific melatonin binding in hamster median eminence and anterior pituitary. Brain Res. 1989, 477, 387–390. [Google Scholar] [CrossRef]
- Vaněček, J.; Klein, D.C. A subpopulation of neonatal gonadotropin-releasing hormone-sensitive pituitary cells is responsive to melatonin. Endocrinology 1993, 133, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.; Messager, S.; Barrett, P.; Hazlerigg, D. Melatonin action in the pituitary: Neuroendocrine synchronizer and developmental modulator? J. Neuroendocrinol. 2003, 15, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.; Bolborea, M. Molecular pathways involved in seasonal body weight and reproductive responses governed by melatonin. J. Pineal Res. 2012, 52, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.R.; Carter, S.N.; Freeman, D.A. Exogenous T3 Elicits Long Day–Like Alterations in Testis Size and the RFamides Kisspeptin and Gonadotropin-Inhibitory Hormone in Short-Day Siberian Hamsters. J. Biol. Rhythm. 2013, 28, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, B.J.; Pyter, L.M.; Kampf-Lassin, A.; Patel, P.N.; Stevenson, T.J. Rapid induction of hypothalamic iodothyronine deiodinase expression by photoperiod and melatonin in juvenile Siberian hamsters (Phodopus sungorus). Endocrinology 2013, 154, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Revel, F.G.; Saboureau, M.; Pévet, P.; Mikkelsen, J.D.; Simonneaux, V. Melatonin regulates type 2 deiodinase gene expression in the Syrian hamster. Endocrinology 2006, 147, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Matsuo, H.; Iigo, M.; Furuse, M.; Korf, H.-W.; Yasuo, S. Melatonin-induced changes in the expression of thyroid hormone-converting enzymes in hypothalamus depend on the timing of melatonin injections and genetic background in mice. Gen. Comp. Endocrinol. 2013, 186, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, S.; Yoshimura, T.; Ebihara, S.; Korf, H.-W. Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology 2007, 148, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, S.; Yoshimura, T.; Ebihara, S.; Korf, H. Photoperiodic Control of TSH-β Expression in the Mammalian Pars Tuberalis has Different Impacts on the Induction and Suppression of the Hypothalamo-Hypopysial Gonadal Axis. J. Neuroendocrinol. 2010, 22, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yasuo, S.; Watanabe, T.; Yamamura, T.; Nakao, N.; Ebihara, S.; Yoshimura, T. Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: Possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology 2004, 145, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Filippa, V.; Penissi, A.; Mohamed, F. Seasonal variations of gonadotropins in the pars distalis male viscacha pituitary. Effect of chronic melatonin treatment. Eur. J. Histochem. 2005, 49, 291. [Google Scholar] [PubMed]
- Messager, S.; Caillol, M.; Martinet, L. Long-term exposure of hypothalamic explants to melatonin alters the release of gonadotrophin releasing hormone and the density of melatonin binding sites in the pars tuberalis of the male mink (Mustela vison). J. Pineal Res. 1999, 26, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, K.; Yoshimura, T. Molecular mechanism regulating seasonality. In Biological Timekeeping: Clocks, Rhythms and Behaviour; Springer: New Delhi, India, 2017; pp. 589–605. [Google Scholar]
- Chemineau, P.; Normant, E.; Ravault, J.; Thimonier, J. Induction and persistence of pituitary and ovarian activity in the out-of-season lactating dairy goat after a treatment combining a skeleton photoperiod, melatonin and the male effect. J. Reprod. Fertil. 1986, 78, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G.; Clarke, I. Photoperiodically-lnduced Cycles in the Secretion of Prolactin in Hypothalamo-Pituitary Disconnected Rams: Evidence for Translation of the Melatonin Signal in the Pituitary Gland. J. Neuroendocrinol. 1994, 6, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Skinner, D.C.; Robinson, J.E. Luteinising hormone secretion from the perifused ovine pars tuberalis and pars distalis: Effects of gonadotropin-releasing hormone and melatonin. Neuroendocrinology 1997, 66, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, U.; Weitzman, E.; Fukushima, D.; Cancel, G.; Rosenfeld, R. Melatonin does not suppress the pituitary luteinizing hormone response to luteinizing hormone-releasing hormone in men. J. Clin. Endocrinol. Metab. 1980, 51, 161. [Google Scholar] [CrossRef] [PubMed]
- Luboshitzky, R.; Levi, M.; Shen-Orr, Z.; Blumenfeld, Z.; Herer, P.; Lavie, P. Long-term melatonin administration does not alter pituitary-gonadal hormone secretion in normal men. Hum. Reprod. 2000, 15, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, A.; Kivelä, A.; Pakarinen, A.; Vakkuri, O. Inverse seasonal relationship between melatonin and ovarian activity in humans in a region with a strong seasonal contrast in luminosity. J. Clin. Endocrinol. Metab. 1987, 65, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, A.; Kauppila, A.; Ylöstalo, P.; Vakkuri, O.; Leppäluoto, J. Seasonal, menstrual and circadian secretions of melatonin, gonadotropins and prolactin in women. Acta Physiol. 1988, 132, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Handa, R.J.; Burgess, L.H.; Kerr, J.E.; O’Keefe, J.A. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994, 28, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Pérez, J.; Casao, A.; González-Arto, M.; Santos Hamilton, T.; Pérez-Pé, R.; Muiño-Blanco, T. Melatonin in sperm biology: Breaking paradigms. Reprod. Domest. Anim. 2014, 49, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.H.C.; Leal, C.L.V.; da Cruz, J.F.; Tan, D.-X.; Reiter, R.J. Role of melatonin on production and preservation of gametes and embryos: A brief review. Anim. Reprod. Sci. 2014, 145, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.A.; Manchester, L.C.; Tan, D.-X. Peripheral reproductive organ health and melatonin: Ready for prime time. Int. J. Mol. Sci. 2013, 14, 7231–7272. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.; Costa, N.; Souza, E.; Gonçalves, A.; Silva, T.; Santana, P.; Santos, A.; Azevedo, P.; Miranda, M.; Santos, S. Melatonin during bovine oocyte maturation and embryonic development improves the quality of in vitro produced embryos. Anim. Reprod. 2017, 14, 165. [Google Scholar]
- Soto-Heras, S.; Roura, M.; Catalá, M.G.; Menéndez-Blanco, I.; Izquierdo, D.; Fouladi-Nashta, A.A.; Paramio, M.T. Beneficial effects of melatonin on in vitro embryo production from juvenile goat oocytes. Reprod. Fertil. Dev. 2018, 30, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Sheng, S.; Sun, Y.; Li, H.; Li, W.-P.; Zhang, C.; Chen, Z.-J. Melatonin levels in follicular fluid as markers for IVF outcomes and predicting ovarian reserve. Reproduction 2017, 153, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Wang, F.; Tian, X.; Ji, P.; Liu, G. Effects of melatonin administration on embryo implantation and offspring growth in mice under different schedules of photoperiodic exposure. Reprod. Biol. Endocrinol. 2017, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tong, J.; Li, W.-P.; Chen, Z.-J.; Zhang, C. Melatonin concentration in follicular fluid is correlated with antral follicle count (AFC) and in vitro fertilization (IVF) outcomes in women undergoing assisted reproductive technology (ART) procedures. Gynecol. Endocrinol. 2017, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.; Rato, L.; Martins, A.; Alves, M.; Oliveira, P. Melatonin and male reproductive health: Relevance of darkness and antioxidant properties. Curr. Mol. Med. 2015, 15, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Bornman, M.; Oosthuizen, J.; Barnard, H.; Schulenburg, G.; Boomker, D.; Reif, S. Melatonin and sperm motility/melatonin und spermatozoenmotilität. Andrologia 1989, 21, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Gwayi, N.; Bernard, R. The effects of melatonin on sperm motility in vitro in Wistar rats. Andrologia 2002, 34, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Fujinoki, M. Melatonin-enhanced hyperactivation of hamster sperm. Reproduction 2008, 136, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Casao, A.; Pérez-Pé, R.; Abecia, J.A.; Forcada, F.; Muiño-Blanco, T.; Cebrián-Pérez, J.Á. The effect of exogenous melatonin during the non-reproductive season on the seminal plasma hormonal profile and the antioxidant defence system of Rasa Aragonesa rams. Anim. Reprod. Sci. 2013, 138, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kokolis, N.; Theodosiadou, E.; Tsantarliotou, M.; Rekkas, C.; Goulas, P.; Smokovitis, A. The effect of melatonin implants on blood testosterone and acrosin activity in spermatozoa of the ram. Andrologia 2000, 32, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Haldar, C. Photoperiodic regulation of melatonin membrane receptor (MT1R) expression and steroidogenesis in testis of adult golden hamster, Mesocricetus auratus. J. Photochem. Photobiol. B Biol. 2014, 140, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Takiguchi, S.; Kashida, S.; Yamagata, Y.; Sugino, N.; Kato, H. Melatonin directly suppresses steroid production by preovulatory follicles in the cyclic hamster. J. Pineal Res. 1998, 25, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, I.; Jacquet, P.; Cortvrindt, R.; Janssen, K.; Smitz, J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology 2006, 228, 333–343. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ma, T.; Shi, J.; Zhang, Z.; Wang, J.; Zhu, K.; Li, Y.; Yang, M.; Song, Y.; Liu, G. Melatonin and its receptor MT1 are involved in the downstream reaction to luteinizing hormone and participate in the regulation of luteinization in different species. J. Pineal Res. 2016, 61, 279–290. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Sosa, G.A.; El-Roos, M.E.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Leska, A.; Dusza, L. Seasonal changes in the hypothalamo-pituitary-gonadal axis in birds. Reprod. Biol. 2007, 7, 99–126. [Google Scholar] [PubMed]
- Mayer, I.; Bornestaf, C.; Borg, B. Melatonin in non-mammalian vertebrates: physiological role in reproduction? Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 515–531. [Google Scholar] [CrossRef]
- Homma, K.; McFarland, L.Z.; Wilson, W.O. Response of the reproductive organs of the Japanese quail to pinealectomy and melatonin injections. Poult. Sci. 1967, 46, 314–319. [Google Scholar] [CrossRef]
- Sayler, A.; Wolfson, A. Influence of the Pineal Gland on Gonadal Maturation in the Japanese Quail 1. Endocrinology 1968, 83, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Storey, C.R.; Nicholls, T.J. Failure of exogenous melatonin to influence the maintenance or dissipation of photorefractoriness in the canary, Serinus canarius. Gen. Comp. Endocrinol. 1978, 34, 468–470. [Google Scholar] [CrossRef]
- Chaturvedi, C. Effect of Melatonin on the Adrenl and Gonad of the Common Mynah Acridtheres tristis. Aust. J. Zool. 1984, 32, 803–809. [Google Scholar] [CrossRef]
- Haldar, C.; Ghosh, M. Annual pineal and testicular cycle in the Indian jungle bush quail, Perdicula asiatica, with reference to the effect of pinealectomy. Gen. Comp. Endocrinol. 1990, 77, 150–157. [Google Scholar] [CrossRef]
- Juss, T.S.; Meddle, S.L.; Servant, R.S.; King, V.M. Melatonin and photoperiodic time measurement in Japanese quail (Coturnix Coturnix japonica). Proc. Biol. Sci. 1993, 254, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Patel, M.; Patel, C. Effects of pineal indoles and parachlorophenylalanine on seasonal reproduction in the pigeon. J. Exp. Biol. 1996, 199, 793–800. [Google Scholar] [PubMed]
- Kumar Maitra, S.; Dey, M. Melatonin does not modulate testicular responsiveness to altered photoperiods. A study during different phases of the annual gonadal cycle in Roseringedparakeets (Psittacula krameri). Biol. Rhythm Res. 1996, 27, 72–86. [Google Scholar] [CrossRef]
- Ohta, M.; Kadota, C.; Konishi, H. A role of melatonin in the initial stage of photoperiodism in the Japanese quail. Biol. Reprod. 1989, 40, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Pelham, R. A Serum Melatonin Rhythm in Chickens and Its Abolition by Pinealectomy 1. Endocrinology 1975, 96, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Cogburn, L.A.; Wilson-Placentra, S.; Letcher, L.R. Influence of pinealectomy on plasma and extrapineal melatonin rhythms in young chickens (Gallus domesticus). Gen. Comp. Endocrinol. 1987, 68, 343–356. [Google Scholar] [CrossRef]
- Vakkuri, O.; Rintamäki, H.; Leppäluoto, J. Plasma and tissue concentrations of melatonin after midnight light exposure and pinealectomy in the pigeon. J. Endocrinol. 1985, 105, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Vakkuri, O.; Rintamäki, H.; Leppäluoto, J. Presence of immunoreactive melatonin in different tissues of the pigeon (Columba livia). Gen. Comp. Endocrinol. 1985, 58, 69–75. [Google Scholar] [CrossRef]
- Gwinner, E.; Hau, M.; Heigl, S. Melatonin: Generation and modulation of avian circadian rhythms. Brain Res. Bull. 1997, 44, 439–444. [Google Scholar] [CrossRef]
- Bentley, G.E. Unraveling the enigma: The role of melatonin in seasonal processes in birds. Microsc. Res. Tech. 2001, 53, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.E. Photoperiodism and reproduction in birds. In Photoperiodism: The Biological Calendar; Oxford University Press: Oxford, UK, 2010; pp. 420–445. [Google Scholar]
- Dawson, A.; King, V.M.; Bentley, G.E.; Ball, G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythm. 2001, 16, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Bentley, G.E.; Tsutsui, K. Neuroendocrine regulation of gonadotropin secretion in seasonally breeding birds. Front. Neurosci. 2013, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.; Follett, B. The blood supply to the pituitary and basal hypothalamus in the Japanese quail (Coturnix Coturnix japonica). J. Anatomy 1969, 104, 227. [Google Scholar]
- Davies, D.; Follett, B. The Neuroendocrine Control of Gonadotrophin Release in the Japanese Quail II. The Role of the Anterior Hypothalamus; The Royal Society: London, UK, 1975; Volume 191, pp. 303–315. [Google Scholar]
- Ohta, M.; Wada, M.; Homma, K. Induction of rapid testicular growth in Japanese quail by phasic electrical stimulation of the hypothalamic photosensitive area. J. Comp. Physiol. A 1984, 154, 583–589. [Google Scholar] [CrossRef]
- Meddle, S.L.; Follett, B.K. Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J. Neurosci. 1997, 17, 8909–8918. [Google Scholar] [PubMed]
- Meddle, S.; Follett, B. Photoperiodic activation of fos-like immunoreactive protein in neurones within the tuberal hypothalamus of Japanese quail. J. Comp. Physiol. A 1995, 176, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Sayler, A.; Wolfson, A. Avian pineal gland: Progonadotropic response in the Japanese quail. Science 1967, 158, 1478–1479. [Google Scholar] [CrossRef] [PubMed]
- Cassone, V.M.; Brooks, D.S. Sites of melatonin action in the brain of the house sparrow, Passer domesticus. J. Exp. Zool. Part A Ecol. Genet. Physiol. 1991, 260, 302–309. [Google Scholar] [CrossRef]
- Yasuo, S.; Watanabe, M.; Okabayashi, N.; Ebihara, S.; Yoshimura, T. Circadian clock genes and photoperiodism: Comprehensive analysis of clock gene expression in the mediobasal hypothalamus, the suprachiasmatic nucleus, and the pineal gland of Japanese quail under various light schedules. Endocrinology 2003, 144, 3742–3748. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.E.; Tucker, S.; Chou, H.; Hau, M.; Perfito, N. Testicular growth and regression are not correlated with Dio2 expression in a wild male songbird, Sturnus vulgaris, exposed to natural changes in photoperiod. Endocrinology 2013, 154, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Perfito, N.; Guardado, D.; Williams, T.D.; Bentley, G.E. Social cues regulate reciprocal switching of hypothalamic Dio2/Dio3 and the transition into final follicle maturation in European starlings (Sturnus vulgaris). Endocrinology 2014, 156, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Nakao, N.; Ono, H.; Yoshimura, T. Thyroid hormones and seasonal reproductive neuroendocrine interactions. Reproduction 2008, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Leclerc, B.; Kosonsiriluk, S.; Mauro, L.; Iwasawa, A.; El Halawani, M. Melanopsin expression in dopamine-melatonin neurons of the premammillary nucleus of the hypothalamus and seasonal reproduction in birds. Neuroscience 2010, 170, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, V.S.; Yamamoto, K.; Ubuka, T.; Bentley, G.E.; Hattori, A.; Tsutsui, K. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology 2010, 151, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Kim, S.; Huang, Y.; Reid, J.; Jiang, J.; Osugi, T.; Chowdhury, V.S.; Tsutsui, K.; Bentley, G.E. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and-II neurons in European starling brain. Endocrinology 2008, 149, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Kumari, Y.; Rani, S.; Tsutsui, K.; Kumar, V. Duration of melatonin regulates seasonal plasticity in subtropical Indian weaver bird, Ploceus philippinus. Gen. Comp. Endocrinol. 2015, 220, 46–54. [Google Scholar]
- Chowdhury, V.S.; Ubuka, T.; Tsutsui, K. Review: Melatonin stimulates the synthesis and release of gonadotropin-inhibitory hormone in birds. Gen. Comp. Endocrinol. 2013, 181, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.E.; Ubuka, T.; McGuire, N.L.; Chowdhury, V.S.; Morita, Y.; Yano, T.; Hasunuma, I.; Binns, M.; Wingfield, J.C.; Tsutsui, K. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen. Comp. Endocrinol. 2008, 156, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, A. Prolactin in avian reproductive cycles. In Hormones and Behaviour in Higher Vertebrates; Springer: Berlin/Heidelberg, Germany, 1983; pp. 375–387. [Google Scholar]
- Ziegler, T.E. Hormones associated with non-maternal infant care: A review of mammalian and avian studies. Folia Primatol. 2000, 71, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, G.L. Effect of prolonged ACTH administration on the gonads of sexually mature chickens. Poult. Sci. 1966, 45, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Deviche, P.; Balthazart, J.; Heyns, W.; Hendrick, J.-C. Endocrine effects of castration followed by androgen replacement and ACTH injections in the male domestic duck (Anas platyrhynchos L.). Gen. Comp. Endocrinol. 1980, 41, 53–61. [Google Scholar] [CrossRef]
- Chaturvedi, C.; Suresh, P. Effects of corticosterone, metapyrone, and ACTH on testicular function at different stages of the breeding cycle in migratory redheaded bunting, Emberiza bruniceps. Gen. Comp. Endocrinol. 1990, 78, 1–11. [Google Scholar] [CrossRef]
- Rząsa, J.; Ewy, Z. Effect of vasotocin and oxytocin on oviposition in the hen. J. Reprod. Fertil. 1970, 21, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, D.A.; Cornett, L.E. The cloned avian neurohypophysial hormone receptors. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 143, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Tomaszycki, M. Oxytocin antagonist treatments alter the formation of pair relationships in zebra finches of both sexes. Horm. Behav. 2012, 62, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.; Dennison, P. Prolactin and luteinizing hormone levels in female turkeys (Meleagris gallopavo) during a photoinduced reproductive cycle and broodiness. Gen. Comp. Endocrinol. 1980, 41, 92–100. [Google Scholar] [CrossRef]
- Sharp, P.J.; Dawson, A.; Lea, R.W. Control of luteinizing hormone and prolactin secretion in birds. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 119, 275–282. [Google Scholar] [CrossRef]
- Sockman, K.W.; Sharp, P.J.; Schwabl, H. Orchestration of avian reproductive effort: An integration of the ultimate and proximate bases for flexibility in clutch size, incubation behaviour, and yolk androgen deposition. Biol. Rev. 2006, 81, 629–666. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.; Goldsmith, A.R. Prolactin and gonadotrophin secretion in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to nesting, incubation, and rearing young. Gen. Comp. Endocrinol. 1982, 48, 213–221. [Google Scholar] [CrossRef]
- Yuan, H.; Tang, F.; Pang, S.F. Binding Characteristics, Regional Distribution and Diurnal Variation of [125I]-Iodomelatonin Binding Sites in the Chicken Brain. J. Pineal Res. 1990, 9, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Kameda, Y.; Miura, M.; Maruyama, S. Effect of pinealectomy on the photoperiod-dependent changes of the specific secretory cells and α-subunit mRNA level in the chicken pars tuberalis. Cell Tissue Res. 2002, 308, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Haldar-Misra, C.; Ghosh, M.; Thapliyal, J. Effect of melatonin on gonads, body weight, and luteinizing hormone (LH) dependent coloration of the Indian finch, Lal munia (Estrilda amandava). Gen. Comp. Endocrinol. 1987, 65, 451–456. [Google Scholar] [CrossRef]
- Rozenboim, I.; Aharony, T.; Yahav, S. The effect of melatonin administration on circulating plasma luteinizing hormone concentration in castrated White Leghorn roosters. Poultry Sci. 2002, 81, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, B.P.; Rani, S. The bird clock: A complex, multi-oscillatory and highly diversified system. Biol. Rhythm Res. 2004, 35, 121–144. [Google Scholar] [CrossRef]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, S.; Watanabe, M.; Tsukada, A.; Takagi, T.; Iigo, M.; Shimada, K.; Ebihara, S.; Yoshimura, T. Photoinducible phase-specific light induction of Cry1 gene in the pars tuberalis of Japanese quail. Endocrinology 2004, 145, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Pauers, M.J.; Kuchenbecker, J.A.; Neitz, M.; Neitz, J. Changes in the colour of light cue circadian activity. Anim. Behav. 2012, 83, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Tang, I.-H.; Murakami, D.M.; Fuller, C.A. Effects of square-wave and simulated natural light-dark cycles on hamster circadian rhythms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 276, R1195–R1202. [Google Scholar] [CrossRef]
- Boulos, Z.; Macchi, M.M. Season-and latitude-dependent effects of simulated twilights on circadian entrainment. J. Biol. Rhythm. 2005, 20, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Perfito, N.; Jeong, S.Y.; Silverin, B.; Calisi, R.M.; Bentley, G.E.; Hau, M. Anticipating spring: Wild populations of great tits (Parus major) differ in expression of key genes for photoperiodic time measurement. PLoS ONE 2012, 7, e34997. [Google Scholar] [CrossRef] [PubMed]
- Ayre, E.A.; Wang, Z.P.; Brown, G.M.; Pang, S.F. Localization and characterization of [125I] iodomelatonin binding sites in duck gonads. J. Pineal Res. 1994, 17, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yang, M.; Zhu, K.; Wang, L.; Song, Y.; Wang, J.; Qin, W.; Xu, Z.; Chen, Y.; Liu, G. Melatonin implantation improved the egg-laying rate and quality in hens past their peak egg-laying age. Sci. Rep. 2016, 6, 39799. [Google Scholar] [CrossRef] [PubMed]
- Greives, T.J.; Kingma, S.A.; Beltrami, G.; Hau, M. Melatonin delays clutch initiation in a wild songbird. Biol. Lett. 2012, 8, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Haldar, C. Environmental factors and annual harderian–pineal–gonadal interrelationship in Indian jungle bush quail, Perdicula asiatica. Gen. Comp. Endocrinol. 1997, 106, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Sudhakumari, C.; Haldar, C.; Senthilkumaran, B. Seasonal changes in adrenal and gonadal activity in the quail, Perdicula asiatica: Involvement of the pineal gland. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 128, 793–804. [Google Scholar] [CrossRef]
- McGuire, N.L.; Koh, A.; Bentley, G.E. The direct response of the gonads to cues of stress in a temperate songbird species is season-dependent. PeerJ 2013, 1, e139. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Haldar, C. Peripheral melatonin modulates seasonal immunity and reproduction of Indian tropical male bird Perdicula asiatica. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Wolfson, A. Lack of an effect of melatonin treatment via silastic capsules on photic-induced gonadal growth and the photorefractory condition in white-throated sparrows. Gen. Comp. Endocrinol. 1978, 34, 471–474. [Google Scholar] [CrossRef]
- Harding, C.F.; Sheridan, K.; Walters, M.J. Hormonal specificity and activation of sexual behavior in male zebra finches. Horm. Behav. 1983, 17, 111–133. [Google Scholar] [CrossRef]
- Ernst, D.K.; Lynn, S.E.; Bentley, G.E. Differential response of GnIH in the brain and gonads following acute stress in a songbird. Gen. Comp. Endocrinol. 2016, 227, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ball, G.F.; Riters, L.V.; Balthazart, J. Neuroendocrinology of song behavior and avian brain plasticity: Multiple sites of action of sex steroid hormones. Front. Neuroendocrinol. 2002, 23, 137–178. [Google Scholar] [CrossRef] [PubMed]
- Cassone, V.M.; Paulose, J.K.; Whitfield-Rucker, M.G.; Peters, J.L. Time’s arrow flies like a bird: Two paradoxes for avian circadian biology. Gen. Comp. Endocrinol. 2009, 163, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Oksche, A. Sensory and glandular elements of the pineal organ. Pineal Gland 1971, 127–146. [Google Scholar]
- Ekström, P.; Meissl, H. Evolution of photosensory pineal organs in new light: The fate of neuroendocrine photoreceptors. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 1679–1700. [Google Scholar] [CrossRef] [PubMed]
- Petit, A. L’épiphyse d’un serpent: Tropidonotus natrix L. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie 1971, 120, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Underwood, H. The pineal and melatonin: Regulators of circadian function in lower vertebrates. Cell. Mol. Life Sci. 1989, 45, 914–922. [Google Scholar] [CrossRef]
- Ooka-Souda, S.; Kadota, T.; Kabasawa, H. The preoptic nucleus: The probable location of the circadian pacemaker of the hagfish, Eptatretus burgeri. Neurosci. Lett. 1993, 164, 33–36. [Google Scholar] [CrossRef]
- Firth, B.T.; Christian, K.A.; Belan, I.; Kennaway, D.J. Melatonin rhythms in the Australian freshwater crocodile (Crocodylus johnstoni): A reptile lacking a pineal complex? J. Comp. Physiol. B 2010, 180, 67. [Google Scholar] [CrossRef] [PubMed]
- Sower, S.A.; Freamat, M.; Kavanaugh, S.I. The origins of the vertebrate hypothalamic–pituitary–gonadal (HPG) and hypothalamic–pituitary–thyroid (HPT) endocrine systems: New insights from lampreys. Gen. Comp. Endocrinol. 2009, 161, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Sower, S.A. The reproductive hypothalamic-pituitary axis in lampreys. In Lampreys: Biology, Conservation and Control; Springer: Dordrecht, The Netherlands, 2015; pp. 305–373. ISBN 94-017-9305-0. [Google Scholar]
- Levey, I.L. Effects of pinealectomy and melatonin injections at different seasons on ovarian activity in the lizard Anolis carolinensis. J. Exp. Zool. Part A Ecol. Genet. Physiol. 1973, 185, 169–174. [Google Scholar] [CrossRef]
- Underwood, H. Annual testicular cycle of the lizard Anolis carolinensis: Effects of pinealectomy and melatonin. J. Exp. Zool. Part A Ecol. Genet. Physiol. 1985, 233, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Thapliyal, J.; Nee Haldar, C.M. Effect of pinealectomy on the photoperiodic gonadal response of the Indian garden lizard, Calotes versicolor. Gen. Comp. Endocrinol. 1979, 39, 79–86. [Google Scholar] [CrossRef]
- Cuellar, H.S. Continuance of circannual reproductive refractoriness in pinealectomized parthenogenetic whiptails (Cnemidophorus uniparens). J. Exp. Zool. Part A Ecol. Genet. Physiol. 1978, 206, 207–214. [Google Scholar] [CrossRef]
- Vu, M.; Trudeau, V.L. Neuroendocrine control of spawning in amphibians and its practical applications. Gen. Comp. Endocrinol. 2016, 234, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Vivien-Roels, B. Effect of environmental temperature and photoperiod on the melatonin levels in the pineal, lateral eye, and plasma of the frog, Rana perezi: Importance of ocular melatonin. Gen. Comp. Endocrinol. 1989, 75, 46–53. [Google Scholar] [CrossRef]
- Isorna, E.; Delgado, M.J.; Guijarro, A.I.; López-Patiño, M.A.; Alonso-Bedate, M.; Alonso-Gómez, A.L. 2-[125 I]-Melatonin binding sites in the central nervous system and neural retina of the frog Rana perezi: Regulation by light and temperature. Gen. Comp. Endocrinol. 2004, 139, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gómez, A.; Tejera, M.; Alonso-Bedate, M.; Delgado, M. Response to pinealectomy and blinding in vitellogenic female frogs (Rana perezi) subjected to high temperature in autumn. Can. J. Physiol. Pharmacol. 1990, 68, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Easley, K.A.; Culley, D.D.; Horseman, N.D.; Penkala, J.E. Environmental influences on hormonally induced spermiation of the bullfrog, Rana catesbeiana. J. Exp. Zool. Part A Ecol. Genet. Physiol. 1979, 207, 407–416. [Google Scholar] [CrossRef]
- Horseman, N.D.; Smith, C.A.; Culley, D.D., Jr. Effects of age and photoperiod on ovary size and condition in bullfrogs (Rana catesbeiana Shaw) (Amphibia, Anura, Ranidae). J. Herpetol. 1978, 12, 287–290. [Google Scholar] [CrossRef]
- Canavero, A.; Arim, M. Clues supporting photoperiod as the main determinant of seasonal variation in amphibian activity. J. Nat. Hist. 2009, 43, 2975–2984. [Google Scholar] [CrossRef]
- Claes, J.M.; Ho, H.-C.; Mallefet, J. Control of luminescence from pygmy shark (Squaliolus aliae) photophores. J. Exp. Biol. 2012, 215, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.I.; Tay, B.-H.; Zheng, L.; Danks, J.A.; Brenner, S.; Foster, R.G.; Collin, S.P.; Hankins, M.W.; Venkatesh, B.; Hunt, D.M. Evolution and functional characterisation of melanopsins in a deep-sea chimaera (Elephant shark, Callorhinchus milii). PLoS ONE 2012, 7, e51276. [Google Scholar] [CrossRef] [PubMed]
- Pierantoni, R.; D’Antonio, M.; Fasano, S. Morpho-functional aspects of the hypothalamus-pituitary-gonadal axis of elasmobranch fishes. In The Reproduction and Development of Sharks, Skates, Rays and Ratfishes; Springer: Dordrecht, The Netherlands, 1993; pp. 187–196. [Google Scholar]
- Falcón, J.; Besseau, L.; Sauzet, S.; Boeuf, G. Melatonin effects on the hypothalamo–pituitary axis in fish. Trends Endocrinol. Metab. 2007, 18, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Falcon, J.; Migaud, H.; Munoz-Cueto, J.A.; Carrillo, M. Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 469–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachmann, A.; Falcon, J.; Knijff, S.; Bolliet, V.; Ali, M. Effects of photoperiod and temperature on rhythmic melatonin secretion from the pineal organ of the white sucker (Catostomus commersoni) in vitro. Gen. Comp. Endocrinol. 1992, 86, 26–33. [Google Scholar] [CrossRef]
- Maitra, S.K.; Chattoraj, A.; Mukherjee, S.; Moniruzzaman, M. Melatonin: A potent candidate in the regulation of fish oocyte growth and maturation. Gen. Comp. Endocrinol. 2013, 181, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C. Melatonin in fish: Circadian rhythm and functions. In Biological Clock in Fish; Science Publishers: Hauppauge, NY, USA, 2010; pp. 71–91. [Google Scholar]
- Gern, W.; Dickhoff, W.W.; Folmar, L.C. Increases in plasma melatonin titers accompanying seawater adaptation of coho salmon (Oncorhynchus kisutch). Gen. Comp. Endocrinol. 1984, 55, 458–462. [Google Scholar] [CrossRef]
- Maitra, S.K.; Hasan, K.N. The Role of Melatonin as a Hormone and an Antioxidant in the Control of Fish Reproduction. Front. Endocrinol. 2016, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Collin, S.P.; Hart, N.S. Vision and photoentrainment in fishes: The effects of natural and anthropogenic perturbation. Integr. Zool. 2015, 10, 15–28. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, D.M. Eco-endo-immunology across avian life history stages. Gen. Comp. Endocrinol. 2013, 190, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Lattin, C.R.; Breuner, C.W.; Romero, L.M. Does corticosterone regulate the onset of breeding in free-living birds? The CORT-Flexibility Hypothesis and six potential mechanisms for priming corticosteroid function. Horm. Behav. 2016, 78, 107–120. [Google Scholar] [PubMed]
- Calisi, R.M. An integrative overview of the role of gonadotropin-inhibitory hormone in behavior: Applying Tinbergen’s four questions. Gen. Comp. Endocrinol. 2014, 203, 95–105. [Google Scholar] [CrossRef] [PubMed]
- De Atenor, M.; De Romero, I.; Brauckmann, E.; Pisano, A.; Legname, A. Effects of the pineal gland and melatonin on the metabolism of oocytes in vitro and on ovulation in Bufo arenarum. J. Exp. Zool. Part A Ecol. Genet. Physiol. 1994, 268, 436–441. [Google Scholar] [CrossRef] [PubMed]
- de Vlaming, V.L.; Sage, M.; Charlton, C.B. The effects of melatonin treatment on gonosomatic index in the teleost, Fundulus similis, and the tree frog, Hyla cinerea. Gen. Comp. Endocrinol. 1974, 22, 433–438. [Google Scholar] [CrossRef]
- Bentley, G.E.; Wilsterman, K.; Ernst, D.K.; Lynn, S.E.; Dickens, M.J.; Calisi, R.M.; Kriegsfeld, L.J.; Kaufer, D.; Geraghty, A.C.; viviD, D. Neural versus gonadal GnIH: Are they independent systems? A mini-review. Integr. Comp. Biol. 2017, 57, 1194–1203. [Google Scholar] [CrossRef] [PubMed]






| Mel1a/MTNR1A | Mel1b/MTNR1B | Mel1C/MTNR1C | NAD(P)H Dehydrogenase Quinone 2/NQO2 (Ribosyldihydronicotinamide Dehydrogenase) | ||||
|---|---|---|---|---|---|---|---|
| MTNR1A_Homo sapiens | NM_005958.4 Homo sapiens melatonin receptor 1A (MTNR1A), mRNA | MTNR1B_Homo sapiens | NM_005959.3 Homo sapiens melatonin receptor 1B (MTNR1B), mRNA | NQO2_Homo sapiens | J02888.1 Human quinone oxidoreductase (NQO2) mRNA, complete cds | ||
| MTNR1A_Gorilla gorilla gorilla | XM_004040725.2 PREDICTED: Gorilla gorilla gorilla melatonin receptor 1A (MTNR1A), mRNA | MTNR1B_Gorilla gorilla gorilla | XM_004051965.2 PREDICTED: Gorilla gorilla gorilla melatonin receptor 1B (MTNR1B), mRNA | NQO2_Gorilla gorilla gorilla | XM_019029756.1 PREDICTED: Gorilla gorilla gorilla NAD(P)H quinone dehydrogenase 2 (NQO2), transcript variant X8, mRNA | ||
| MTNR1A_Macaca mulatta | XM_001090972.3 PREDICTED: Macaca mulatta melatonin receptor 1A (MTNR1A), mRNA | MTNR1B_Macaca mulatta | XM_001084265.3 PREDICTED: Macaca mulatta melatonin receptor 1B (MTNR1B), mRNA | NQO2_Macaca mulatta | XM_015135430.1 PREDICTED: Macaca mulatta NAD(P)H dehydrogenase, quinone 2 (NQO2), transcript variant X3, mRNA | ||
| MTNR1A_Marmota marmota marmota | XM_015486060.1 PREDICTED: Marmota marmota marmota melatonin receptor 1A (Mtnr1a), mRNA | MTNR1B_Marmota marmota marmota | XM_015489993.1 PREDICTED: Marmota marmota marmota melatonin receptor 1B (Mtnr1b), mRNA | NQO2_Marmota marmota marmota | XM_015507543.1 PREDICTED: Marmota marmota marmota NAD(P)H dehydrogenase, quinone 2 (Nqo2), transcript variant X2, mRNA | ||
| MTNR1A_Heterocephalus glaber | XM_004853058.2 PREDICTED: Heterocephalus glaber melatonin receptor 1A (Mtnr1a), mRNA | MTNR1B_Heterocephalus glaber | XM_004838123.1 PREDICTED: Heterocephalus glaber melatonin receptor 1B (Mtnr1b), mRNA | NQO2_Heterocephalus glaber | XM_013073256.1 PREDICTED: Heterocephalus glaber NAD(P)H dehydrogenase, quinone 2 (Nqo2), transcript variant X2, mRNA | ||
| MTNR1A_Peromyscus maniculatus | XM_006970866.1 PREDICTED: Peromyscus maniculatus bairdii melatonin receptor 1A (Mtnr1a), mRNA | MTNR1B_Peromyscus maniculatus bairdii | XM_006990545.1 PREDICTED: Peromyscus maniculatus bairdii melatonin receptor 1B (Mtnr1b), mRNA | NQO2_Peromyscus maniculatus bairdii | XM_006983508.2 PREDICTED: Peromyscus maniculatus bairdii NAD(P)H dehydrogenase, quinone 2 (Nqo2), mRNA | ||
| Mel1a_Phodopus sungorus | U14110.1 Phodopus sungorus melatonin receptor Mel-1a mRNA, complete cds | Mel1b_Phodopus sungorus | U57555.1 Phodopus sungorus Mel1b melatonin receptor mRNA, partial cds | ||||
| MTNR1A_Mus musculus | NM_008639.2 Mus musculus melatonin receptor 1A (Mtnr1a), mRNA | MTNT1B_Mus musculus | NM_145712.2 Mus musculus melatonin receptor 1B (Mtnr1b), mRNA | NAD(P)Hdehydrogenase_ quinone2_Mus musculus | BC027629.1 Mus musculus NAD(P)H dehydrogenase, quinone 2, mRNA (cDNA clone MGC:41088 IMAGE:1225122), complete cds | ||
| MTNR1A_Cricetulus griseus | XM_007617174.2 PREDICTED: Cricetulus griseus melatonin receptor 1A (Mtnr1a), mRNA | Mel1B_Cricetulus griseus | XM_007636225.1 PREDICTED: Cricetulus griseus melatonin receptor type 1B-like (LOC103163272), mRNA | NQO2_Cricetulus griseus | XM_007638944.2 PREDICTED: Cricetulus griseus NAD(P)H quinone dehydrogenase 2 (Nqo2), transcript variant X2, mRNA | ||
| Mel1a_Mesocricetus auratus | AF061158.1 Mesocricetus auratus melatonin receptor Mel1a mRNA, partial cds | Mel1b_Mesocricetus auratus | AY145849 Mesocricetus auratus Mel1b melatonin receptor pseudogene, partial sequence | NQO2_Mesocricetus auratus | XM_013110593.1 PREDICTED: Mesocricetus auratus NAD(P)H dehydrogenase, quinone 2 (Nqo2), transcript variant X1, mRNA | ||
| MTNR1A_Rattus norvegicus | NM_053676.2 Rattus norvegicus melatonin receptor 1A (Mtnr1a), mRNA | MTNR1B_Rattus norvegicus | NM_001100641 Rattus norvegicus melatonin receptor 1B (Mtnr1b), mRNA | NAD(P)Hdehydrogenase_ quinone2_Rattus norvegicus | BC079157.1 Rattus norvegicus NAD(P)H dehydrogenase, quinone 2, mRNA (cDNA clone MGC:94180 IMAGE:7128990), complete cds | ||
| MTNR1A_Gallus gallus | NM_205362.1 Gallus gallus melatonin receptor 1A (MTNR1A), mRNA | MTNR1B_Gallus gallus | NM_001293103.1 Gallus gallus melatonin receptor 1B (MTNR1B), mRNA | NQO2_Gallusgallus | HAEL01011471.1 TSA: Gallus Gallus, breed ISA Brown, contig c17404/1/1559, transcribed RNA sequence | ||
| Mel1A_Coturnix japonica | XM_015862146.1 PREDICTED: Coturnix japonica melatonin receptor type 1A (LOC107313685), mRNA | MTNR1B_Coturnix japonica | XM_015852347.1 PREDICTED: Coturnix japonica melatonin receptor 1B (MTNR1B), transcript variant X1, mRNA | NQO2_Coturnix japonica | XM_015854496.1 PREDICTED: Coturnix japonica NAD(P)H dehydrogenase, quinone 2 (NQO2), mRNA | ||
| MTNR1A_Meleagris gallopavo | XM_003205776.3 PREDICTED: Meleagris gallopavo melatonin receptor 1A (MTNR1A), mRNA | Mel1B_Meleagris gallopavo | XM_010706129.2 PREDICTED: Meleagris gallopavo melatonin receptor type 1B (LOC104909381), mRNA | NQO2_Meleagris gallopavo | XM_010708413.2 PREDICTED: Meleagris gallopavo NAD(P)H quinone dehydrogenase 2 (NQO2), mRNA | ||
| MTNR1A_Sturnus vulgaris | XM_014873402.1 PREDICTED: Sturnus vulgaris melatonin receptor 1A (MTNR1A), mRNA | MTNR1B_Sturnus vulgaris | XM_014877499.1 PREDICTED: Sturnus vulgaris melatonin receptor 1B (MTNR1B), mRNA | NQO2_Sturnus vulgaris | XM_014872375.1 PREDICTED: Sturnus vulgaris NAD(P)H dehydrogenase, quinone 2 (NQO2), mRNA | ||
| MTNR1A_Taeniopygia guttata | NM_001048257.1 Taeniopygia guttata melatonin receptor 1A (MTNR1A), mRNA | MTNR1B_Taeniopygia guttata | NM_001048258.1 Taeniopygia guttata melatonin receptor 1B (MTNR1B), mRNA | NAD(P)Hdehydrogenase_ quinone2_Taeniopygia guttata | DQ216354.1 Taeniopygia guttata clone 0065P0009D12 putative NAD(P)H dehydrogenase quinone 2 variant 2 mRNA, complete cds | ||
| MTNR1A_Columba livia | XM_005500227 PREDICTED: Columba livia melatonin receptor 1A (MTNR1A), mRNA | Mel1B_Columba livia | XM_005512954.2 PREDICTED: Columba livia melatonin receptor type 1B (LOC102095424), mRNA | ||||
| MTNR1A_Parus major | XM_019006224 PREDICTED: Parus major melatonin receptor 1A (MTNR1A), mRNA | MTNR1B_Parus major | XM_015647149 PREDICTED: Parus major melatonin receptor 1B (MTNR1B), mRNA | NQO2_Parus major | XM_015616869.1 PREDICTED: Parus major NAD(P)H quinone dehydrogenase 2 (NQO2), partial mRNA | ||
| MTNR1A_Alligator sinensis | XM_014521485 PREDICTED: Alligator sinensis melatonin receptor 1A (MTNR1A), mRNA | Mel1B_Alligator sinensis | XM_006021055.1 PREDICTED: Alligator sinensis melatonin receptor 1B (MTNR1B), partial mRNA | NQO2_Alligator sinensis | XM_014519268.1 PREDICTED: Alligator sinensis NAD(P)H dehydrogenase, quinone 2 (NQO2), transcript variant X1, mRNA | ||
| MTNR1A_Alligator mississippiensis | XM_006262998 PREDICTED: Alligator mississippiensis melatonin receptor 1A (MTNR1A), transcript variant X1, mRNA | Mel1B_Alligator mississippiensis | XM_006276749.3 PREDICTED: Alligator mississippiensis melatonin receptor type 1B (LOC102567490), partial mRNA | NQO2_Alligator mississippiensis | XM_019490389.1 PREDICTED: Alligator mississippiensis NAD(P)H quinone dehydrogenase 2 (NQO2), transcript variant X2, mRNA | ||
| MTNR1A_Chelonia mydas | XM_007062677 PREDICTED: Chelonia mydas melatonin receptor type 1A-like (LOC102946412), mRNA | Mel1B_Chelonia mydas | XM_007072335.1 PREDICTED: Chelonia mydas melatonin receptor type 1B-like (LOC102947942), mRNA | ||||
| MTNR1A_Pelodiscus sinensis | XM_006128505 PREDICTED: Pelodiscus sinensis melatonin receptor 1A (MTNR1A), mRNA | Mel1B_Pelodiscus sinensis | XM_014577564.1 PREDICTED: Pelodiscus sinensis melatonin receptor type 1B (LOC102444642), mRNA | NQO2_Pelodiscus sinensis | XM_014581445.1 PREDICTED: Pelodiscus sinensis NAD(P)H dehydrogenase, quinone 2 (NQO2), mRNA | ||
| Mel1A_Xenopus laevis | XM_018243177 PREDICTED: Xenopus laevis melatonin receptor type 1A (LOC108706613), mRNA | Mel1B_Xenopus laevis | XM_018250356 PREDICTED: Xenopus laevis melatonin receptor type 1B (LOC108710014), mRNA | Mel1Ca_Xenopus laevis | U67880 Xenopus laevis Mel-1c(a) melatonin receptor long mRNA, complete cds | ||
| Mel1Cb_Xenopus laevis | U67882 Xenopus laevis Mel-1c(b) melatonin receptor long mRNA, complete cds | ||||||
| MTNR1C_Xenopus laevis | KF486518 Xenopus laevis melatonin receptor type 1C (MTNR1C) mRNA, complete cds, alternatively spliced | ||||||
| MTNR1A_Xenopus tropicalis | XM_002940864.3 PREDICTED: Xenopus (Silurana) tropicalis melatonin receptor 1A (mtnr1a), mRNA [removed from NCBI] | MTNR1B_Xenopus tropicalis | XM_004920744 PREDICTED: Xenopus tropicalis melatonin receptor 1B (mtnr1b), partial mRNA | MTNR1C_Xenopus tropicalis | XM_004916882 PREDICTED: Xenopus tropicalis melatonin receptor type 1C (mtnr1c), mRNA | ||
| MTNR1A_Nanorana parkeri | XM_018563328 PREDICTED: Nanorana parkeri melatonin receptor 1A (MTNR1A), mRNA | Mel1B_Nanorana parkeri | XM_018558631 PREDICTED: Nanorana parkeri melatonin receptor type 1B (LOC108788772), mRNA | Mel1C_Nanorana parkeri | XM_018552598 PREDICTED: Nanorana parkeri melatonin receptor type 1C (LOC108783900), mRNA | ||
| Mtnr1Aa_Danio rerio | NM_131393 Danio rerio melatonin receptor 1A a (mtnr1aa), mRNA | Mel1Ba_Danio rerio | BC163408 Danio rerio melatonin receptor type 1B a, mRNA (cDNA clone MGC:194859 IMAGE:9038309), complete cds | MTNR1C_Danio rerio | NM_001161484 Danio rerio melatonin receptor 1C (mtnr1c), mRNA | ||
| Mtnr1Ab_Danio rerio | XM_688989 PREDICTED: Danio rerio melatonin receptor 1A b (mtnr1ab), mRNA | ||||||
| MTNR1A_Pygocentrus nattereri | XM_017704098 PREDICTED: Pygocentrus nattereri melatonin receptor 1A (mtnr1a), transcript variant X1, mRNA | Mel1B_Pygocentrus nattereri | XM_017691296.1 PREDICTED: Pygocentrus nattereri melatonin receptor type 1B-B (LOC108423763), mRNA | Mel1Clike_Pygocentrus nattereri | XM_017713350 PREDICTED: Pygocentrus nattereri melatonin receptor type 1C-like (LOC108436701), mRNA | RibosyldihydronicotinamideDehydrogenase_Pygocentrus nattereri | XM_017684980.1 PREDICTED: Pygocentrus nattereri ribosyldihydronicotinamide dehydrogenase [quinone]-like (LOC108412776), mRNA |
| Mel1C_Pygocentrus nattereri | XM_017721904 PREDICTED: Pygocentrus nattereri melatonin receptor type 1C-like (LOC108442058), mRNA | ||||||
| Mel1Alike_Latimeria chalumnae | XM_014496132 PREDICTED: Latimeria chalumnae melatonin receptor type 1A-like (LOC102351371), partial mRNA | Mel1B_Latimeria chalumnae | XM_014492552 PREDICTED: Latimeria chalumnae melatonin receptor type 1B (LOC102361192), transcript variant X2, mRNA | ||||
| Proximate |
Mechanism
|
| Ultimate |
Adaptive Value
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
ViviD, D.; Bentley, G.E. Seasonal Reproduction in Vertebrates: Melatonin Synthesis, Binding, and Functionality Using Tinbergen’s Four Questions. Molecules 2018, 23, 652. https://doi.org/10.3390/molecules23030652
ViviD D, Bentley GE. Seasonal Reproduction in Vertebrates: Melatonin Synthesis, Binding, and Functionality Using Tinbergen’s Four Questions. Molecules. 2018; 23(3):652. https://doi.org/10.3390/molecules23030652
Chicago/Turabian StyleViviD, Dax, and George E. Bentley. 2018. "Seasonal Reproduction in Vertebrates: Melatonin Synthesis, Binding, and Functionality Using Tinbergen’s Four Questions" Molecules 23, no. 3: 652. https://doi.org/10.3390/molecules23030652
APA StyleViviD, D., & Bentley, G. E. (2018). Seasonal Reproduction in Vertebrates: Melatonin Synthesis, Binding, and Functionality Using Tinbergen’s Four Questions. Molecules, 23(3), 652. https://doi.org/10.3390/molecules23030652