Anomeric O-Functionalization of Carbohydrates for Chemical Conjugation to Vaccine Constructs
Abstract
:1. Introduction
1.1. Emergence of Glycomics
1.2. Overview of Glycosylation Principles
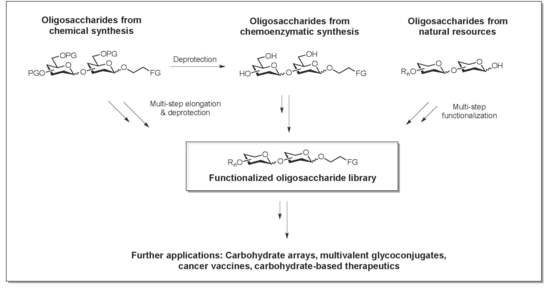
1.3. Elements of Carbohydrate-Based Vaccine Research
2. Stereoselective Anomeric Functionalization for Chemical Conjugation
2.1. Anomeric Functionalization with Terminal Alkenyl Linkers
Diversification of the Alkene Linker Functionality for Carbohydrate Conjugation to Carriers
2.2. O-Anomeric Functionalization with Terminal Azide Linkers
2.2.1. O-Anomeric Functionalization with TMO Addition: Stepwise Introduction of Azide
2.2.2. Multivalent Conjugation Strategies Using Azide-terminated Linkers
3. Functionalized Sugars and Glycan Arrays
4. Conclusions
Funding
Conflicts of Interest
References
- Richard, S.R. New Methods for the Synthesis of Glycosides and Oligosaccharides—Are There Alternatives to the Koenigs-Knorr Method? [New Synthetic Methods (56)]. Angew. Chem. Int. Ed. Engl. 1986, 25, 212–235. [Google Scholar] [CrossRef]
- Werz, D.B.; Ranzinger, R.; Herget, S.; Adibekian, A.; von der Lieth, C.W.; Seeberger, P.H. Exploring the structural diversity of mammalian carbohydrates (“glycospace”) by statistical databank analysis. ACS Chem. Biol. 2007, 2, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Cavalli-Sforza, L.L. The Human Genome Diversity Project: Past, present and future. Nat. Rev. Genet. 2005, 6, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. The human genome project: Past, present, and future. Science 1990, 248, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H. Protein glycosylation: New challenges and opportunities. J. Org. Chem. 2005, 70, 4219–4225. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Wong, C.H. Chemistry and glycobiology. Chem. Commun. 2011, 47, 6201–6207. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.G. Synthesis of glycoproteins. Chem. Rev. 2002, 102, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Sharon, N. Historical Background and Overview. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor: New York, NY, USA, 2009. [Google Scholar]
- Council, N.R. Transforming Glycoscience: A Roadmap for the Future; The National Academies Press: Washington, DC, USA, 2012; 208p. [Google Scholar] [CrossRef]
- Song, X.; Ju, H.; Lasanajak, Y.; Kudelka, M.R.; Smith, D.F.; Cummings, R.D. Oxidative release of natural glycans for functional glycomics. Nat. Methods 2016, 13, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirabayashi, J. Lectin-based structural glycomics: Glycoproteomics and glycan profiling. Glycoconj. J. 2004, 21, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Prieto, P.A. Special considerations for glycolipids and their purification. Curr. Protoc. Mol. Biol. 2001, 22. [Google Scholar] [CrossRef]
- Linhardt, R.J.; Bazin, H.G. Separation and Purification of Carbohydrates. In Glycoscience: Chemistry and Chemical Biology I–III; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 63–74. [Google Scholar]
- Zhu, X.; Schmidt, R.R. New principles for glycoside-bond formation. Angew. Chem. Int. Ed. Engl. 2009, 48, 1900–1934. [Google Scholar] [CrossRef] [PubMed]
- Galonic, D.P.; Gin, D.Y. Chemical glycosylation in the synthesis of glycoconjugate antitumour vaccines. Nature 2007, 446, 1000–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser-Reid, B.; Lopez, J.C. Armed-disarmed effects in carbohydrate chemistry: History, synthetic and mechanistic studies. Top. Curr. Chem. 2011, 301, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Fraser-Reid, B.; Wu, Z.; Udodong, U.E.; Ottosson, H. Armed/disarmed effects in glycosyl donors: Rationalization and sidetracking. J. Org. Chem. 1990, 55, 6068–6070. [Google Scholar] [CrossRef]
- Guo, J.; Ye, X.S. Protecting groups in carbohydrate chemistry: Influence on stereoselectivity of glycosylations. Molecules 2010, 15, 7235–7265. [Google Scholar] [CrossRef] [PubMed]
- Mulani, S.K.; Hung, W.C.; Ingle, A.B.; Shiau, K.S.; Mong, K.K. Modulating glycosylation with exogenous nucleophiles: An overview. Org. Biomol. Chem. 2014, 12, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Bubb, W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts Magn. Reson. A 2003, 19, 1–19. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wong, C.H. Programmable one-pot glycosylation. Top. Curr. Chem. 2011, 301, 223–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ollmann, I.R.; Ye, X.-S.; Wischnat, R.; Baasov, T.; Wong, C.-H. Programmable One-Pot Oligosaccharide Synthesis. J. Am. Chem. Soc. 1999, 121, 734–753. [Google Scholar] [CrossRef]
- Huang, X.; Huang, L.; Wang, H.; Ye, X.S. Iterative one-pot synthesis of oligosaccharides. Angew. Chem. Int. Ed. Engl. 2004, 43, 5221–5224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, X.S.; Zhang, L.H. Oligosaccharide assembly by one-pot multi-step strategy. Org. Biomol. Chem. 2007, 5, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H. Automated oligosaccharide synthesis. Chem. Soc. Rev. 2008, 37, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Hung, S.C.; Wu, C.Y.; Wong, C.H. Toward automated oligosaccharide synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 11872–11923. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Gervay-Hague, J. Synthesis of partially O-acetylated N-acetylneuraminic acid using regioselective silyl exchange technology. Org. Lett. 2014, 16, 5044–5047. [Google Scholar] [CrossRef] [PubMed]
- Witschi, M.A.; Gervay-Hague, J. Selective acetylation of per-O-TMS-protected monosaccharides. Org. Lett. 2010, 12, 4312–4315. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.W.; Schombs, M.W.; Witschi, M.A.; Gervay-Hague, J. Regioselective silyl/acetate exchange of disaccharides yields advanced glycosyl donor and acceptor precursors. J. Org. Chem. 2013, 78, 9677–9688. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.W.; Schombs, M.W.; Gervay-Hague, J. Integrating ReSET with glycosyl iodide glycosylation in step-economy syntheses of tumor-associated carbohydrate antigens and immunogenic glycolipids. J. Org. Chem. 2014, 79, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Caulfield, T.; Kataoka, H.; Kumazawa, T. A practical and enantioselective synthesis of glycosphingolipids and related compounds. Total synthesis of globotriaosylceramide (Gb3). J. Am. Chem. Soc. 1988, 110, 7910–7912. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Caulfield, T.J.; Katoaka, H. Total synthesis of globotriaosylceramide (Gb3) and lysoglobotriaosylceramide (lysoGb3). Carbohydr. Res. 1990, 202, 177–191. [Google Scholar] [CrossRef]
- Feng, D.; Shaikh, A.S.; Wang, F. Recent Advance in Tumor-associated Carbohydrate Antigens (TACAs)-based Antitumor Vaccines. ACS Chem. Biol. 2016, 11, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Ye, X.S. Carbohydrate-based cancer vaccines: Target cancer with sugar bullets. Glycoconj. J. 2012, 29, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Astronomo, R.D.; Burton, D.R. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 2010, 9, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Pashkuleva, I.; Reis, R.L. Sugars: Burden or biomaterials of the future? J. Mater. Chem. 2010, 20, 8803–8818. [Google Scholar] [CrossRef] [Green Version]
- Smoot, J.T.; Demchenko, A.V. Oligosaccharide synthesis: From conventional methods to modern expeditious strategies. Adv. Carbohydr. Chem. Biochem. 2009, 62, 161–250. [Google Scholar] [CrossRef] [PubMed]
- Shiao, T.C.; Roy, R. Glycodendrimers as functional antigens and antitumor vaccines. New J. Chem. 2012, 36, 324–339. [Google Scholar] [CrossRef]
- Adamo, R.; Nilo, A.; Castagner, B.; Boutureira, O.; Berti, F.; Bernardes, G.J. Synthetically defined glycoprotein vaccines: Current status and future directions. Chem. Sci. 2013, 4, 2995–3008. [Google Scholar] [CrossRef] [PubMed]
- Galan, M.C.; Dumy, P.; Renaudet, O. Multivalent glyco(cyclo)peptides. Chem. Soc. Rev. 2013, 42, 4599–4612. [Google Scholar] [CrossRef] [PubMed]
- Branson, T.R.; Turnbull, W.B. Bacterial toxin inhibitors based on multivalent scaffolds. Chem. Soc. Rev. 2013, 42, 4613–4622. [Google Scholar] [CrossRef] [PubMed]
- Horlacher, T.; Seeberger, P.H. Carbohydrate arrays as tools for research and diagnostics. Chem. Soc. Rev. 2008, 37, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Mootoo, D.R.; Date, V.; Fraser-Reid, B. n-Pentenyl glycosides permit the chemospecific liberation of the anomeric center. J. Am. Chem. Soc. 1988, 110, 2662–2663. [Google Scholar] [CrossRef]
- Fraser-Reid, B.; Udodong, U.E.; Wu, Z.; Ottosson, H.; Merritt, J.R.; Rao, C.S.; Roberts, C.; Madsen, R. n-Pentenyl Glycosides in Organic Chemistry: A Contemporary Example of Serendipity. Synlett 1992, 1992, 927–942. [Google Scholar] [CrossRef]
- Rodebaugh, R.; Fraser-Reid, B. Evidence for cyclic bromonium ion transfer in electrophilic bromination of alkenes: Reaction of ω-alkenyl glycosides with aqueous N-bromosuccinimide. Tetrahedron 1996, 52, 7663–7678. [Google Scholar] [CrossRef]
- Clausen, M.H.; Jorgensen, M.R.; Thorsen, J.; Madsen, R. A strategy for chemical synthesis of selectively methyl-esterified oligomers of galacturonic acid. J. Chem. Soc. Perkin Trans. 2001, 1, 543–551. [Google Scholar] [CrossRef]
- Yamada, A.; Hatano, K.; Matsuoka, K.; Koyama, T.; Esumi, Y.; Koshino, H.; Hino, K.; Nishikawa, K.; Natori, Y.; Terunuma, D. Syntheses and Vero toxin-binding activities of carbosilane dendrimers periphery-functionalized with galabiose. Tetrahedron 2006, 62, 5074–5083. [Google Scholar] [CrossRef]
- Allen, J.R.; Danishefsky, S.J. New Applications of the n-Pentenyl Glycoside Method in the Synthesis and Immunoconjugation of Fucosyl GM1: A Highly Tumor-Specific Antigen Associated with Small Cell Lung Carcinoma. J. Am. Chem. Soc. 1999, 121, 10875–10882. [Google Scholar] [CrossRef]
- Hsieh, S.Y.; Jan, M.D.; Patkar, L.N.; Chen, C.T.; Lin, C.C. Synthesis of a carboxyl linker containing Pk trisaccharide. Carbohydr. Res. 2005, 340, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.B.; Plante, O.J.; Melean, L.G.; Seeberger, P.H. Solid-phase oligosaccharide synthesis: Preparation of complex structures using a novel linker and different glycosylating agents. Org. Lett. 1999, 1, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H.; Haase, W.C. Solid-phase oligosaccharide synthesis and combinatorial carbohydrate libraries. Chem. Rev. 2000, 100, 4349–4394. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H. Automated carbohydrate synthesis to drive chemical glycomics. Chem. Commun. 2003, 10, 1115–1121. [Google Scholar] [CrossRef]
- Konradsson, P.; Mootoo, D.R.; McDevitt, R.E.; Fraser-Reid, B. Iodonium ion generated in situ from N-iodosuccinimide and trifluoromethanesulphonic acid promotes direct linkage of ‘disarmed’ pent-4-enyl glycosides. J. Chem. Soc. Chem. Commun. 1990. [Google Scholar] [CrossRef]
- Love, K.R.; Andrade, R.B.; Seeberger, P.H. Linear Synthesis of a Protected H-Type II Pentasaccharide Using Glycosyl Phosphate Building Blocks. J. Org. Chem. 2001, 66, 8165–8176. [Google Scholar] [CrossRef] [PubMed]
- Bosse, F.; Marcaurelle, L.A.; Seeberger, P.H. Linear synthesis of the tumor-associated carbohydrate antigens Globo-H, SSEA-3, and Gb3. J. Org. Chem. 2002, 67, 6659–6670. [Google Scholar] [CrossRef] [PubMed]
- Melean, L.G.; Love, K.R.; Seeberger, P.H. Toward the automated solid-phase synthesis of oligoglucosamines: Systematic evaluation of glycosyl phosphate and glycosyl trichloroacetimidate building blocks. Carbohydr. Res. 2002, 337, 1893–1916. [Google Scholar] [CrossRef]
- Oberli, M.A.; Bindschadler, P.; Werz, D.B.; Seeberger, P.H. Synthesis of a hexasaccharide repeating unit from Bacillus anthracis vegetative cell walls. Org. Lett. 2008, 10, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Matsuoka, K.; Furuike, T.; Ishii, S.; Kurita, K.; Nishimura, K.M. Synthetic glycoconjugates. 2. n-Pentenyl glycosides as convenient mediators for the syntheses of new types of glycoprotein models. Macromolecules 1991, 24, 4236–4241. [Google Scholar] [CrossRef]
- Danishefsky, S.J.; Bilodeau, M.T. Glycals in Organic Synthesis: The Evolution of Comprehensive Strategies for the Assembly of Oligosaccharides and Glycoconjugates of Biological Consequence. Angew. Chem. Int. Ed. Engl. 1996, 35, 1380–1419. [Google Scholar] [CrossRef]
- Buskas, T.; Söderberg, E.; Konradsson, P.; Fraser-Reid, B. Use of n-Pentenyl Glycosides as Precursors to Various Spacer Functionalities. J. Org. Chem. 2000, 65, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Bremer, E.G.; Levery, S.B.; Sonnino, S.; Ghidoni, R.; Canevari, S.; Kannagi, R.; Hakomori, S. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J. Biol. Chem. 1984, 259, 14773–14777. [Google Scholar] [PubMed]
- Slovin, S.F.; Ragupathi, G.; Adluri, S.; Ungers, G.; Terry, K.; Kim, S.; Spassova, M.; Bornmann, W.G.; Fazzari, M.; Dantis, L.; et al. Carbohydrate vaccines in cancer: Immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc. Natl. Acad. Sci. USA 1999, 96, 5710–5715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danishefsky, S.J.; Allen, J.R. From the Laboratory to the Clinic: A Retrospective on Fully Synthetic Carbohydrate-Based Anticancer Vaccines Frequently used abbreviations are listed in the appendix. Angew. Chem. Int. Ed. Engl. 2000, 39, 836–863. [Google Scholar] [CrossRef]
- Wilson, R.M.; Danishefsky, S.J. A vision for vaccines built from fully synthetic tumor-associated antigens: From the laboratory to the clinic. J. Am. Chem. Soc. 2013, 135, 14462–14472. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.J.; Park, T.K.; Hu, S.; Abrampah, K.; Zhang, S.; Livingston, P.; Danishefsky, S.J. Defining the Molecular Recognition of MBr1 (Human Breast Cancer) Antigen by the MBr1 Antibody through Probe Structures Prepared by Total Synthesis. J. Org. Chem. 1995, 60, 7716–7717. [Google Scholar] [CrossRef]
- Allen, J.R.; Allen, J.G.; Zhang, X.F.; Williams, L.J.; Zatorski, A.; Ragupathi, G.; Livingston, P.O.; Danishefsky, S.J. A second generation synthesis of the MBr1 (globo-H) breast tumor antigen: New application of the n-pentenyl glycoside method for achieving complex carbohydrate protein linkages. Chemistry 2000, 6, 1366–1375. [Google Scholar] [CrossRef]
- Bilodeau, M.T.; Park, T.K.; Hu, S.; Randolph, J.T.; Danishefsky, S.J.; Livingston, P.O.; Zhang, S. Total Synthesis of a Human Breast Tumor Associated Antigen. J. Am. Chem. Soc. 1995, 117, 7840–7841. [Google Scholar] [CrossRef]
- Dziadek, S.; Jacques, S.; Bundle, D.R. A novel linker methodology for the synthesis of tailored conjugate vaccines composed of complex carbohydrate antigens and specific TH-cell peptide epitopes. Chemistry 2008, 14, 5908–5917. [Google Scholar] [CrossRef] [PubMed]
- Daskhan, G.C.; Tran, H.T.; Meloncelli, P.J.; Lowary, T.L.; West, L.J.; Cairo, C.W. Construction of Multivalent Homo- and Heterofunctional ABO Blood Group Glycoconjugates Using a Trifunctional Linker Strategy. Bioconjug. Chem. 2018, 29, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Slaney, A.M.; Dijke, I.E.; Jeyakanthan, M.; Li, C.; Zou, L.; Plaza-Alexander, P.; Meloncelli, P.J.; Bau, J.A.; Allan, L.L.; Lowary, T.L.; et al. Conjugation of A and B Blood Group Structures to Silica Microparticles for the Detection of Antigen-Specific B Cells. Bioconjug. Chem. 2016, 27, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. Engl. 2009, 48, 6974–6998. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef] [Green Version]
- Ramil, C.P.; Lin, Q. Bioorthogonal chemistry: Strategies and recent developments. Chem. Commun. 2013, 49, 11007–11022. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Bryan, M.C.; Blixt, O.; Paulson, J.C.; Wong, C.H. Synthesis of sugar arrays in microtiter plate. J. Am. Chem. Soc. 2002, 124, 14397–14402. [Google Scholar] [CrossRef] [PubMed]
- Bryan, M.C.; Fazio, F.; Lee, H.K.; Huang, C.Y.; Chang, A.; Best, M.D.; Calarese, D.A.; Blixt, O.; Paulson, J.C.; Burton, D.; et al. Covalent display of oligosaccharide arrays in microtiter plates. J. Am. Chem. Soc. 2004, 126, 8640–8641. [Google Scholar] [CrossRef] [PubMed]
- Brase, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic azides: An exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. Engl. 2005, 44, 5188–5240. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, H.; Wittman, V. Azides in Carbohydrate Chemistry. In Organic Azides; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 469–490. [Google Scholar]
- Deng, S.; Gangadharmath, U.; Chang, C.W. Sonochemistry: A powerful way of enhancing the efficiency of carbohydrate synthesis. J. Org. Chem. 2006, 71, 5179–5185. [Google Scholar] [CrossRef] [PubMed]
- Michalik, D.; Vliegenthart, J.F.G.; Kamerling, J.P. Chemoenzymic synthesis of oligosaccharide fragments of the capsular polysaccharide of Streptococcus pneumoniae type 14. J. Chem. Soc. Perkin Trans. 2002, 1, 1973–1981. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Kinzy, W. Anomeric-oxygen activation for glycoside synthesis: The trichloroacetimidate method. Adv. Carbohydr. Chem. Biochem. 1994, 50, 21–123. [Google Scholar] [PubMed]
- Yu, B.; Sun, J. Glycosylation with glycosyl N-phenyltrifluoroacetimidates (PTFAI) and a perspective of the future development of new glycosylation methods. Chem. Commun. 2010, 46, 4668–4679. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Hsieh, H.W.; Tsai, C.M.; Hsu, C.W.; Wang, S.G.; Wu, K.J.; Lin, K.I.; Lin, C.H. Synthesis and characterization of sulfated Gal-beta-1,3/4-GlcNAc disaccharides through consecutive protection/glycosylation steps. Chem. Asian J. 2013, 8, 1536–1550. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.M.; Tu, Z.; Jan, H.M.; Pan, J.F.; Lin, C.H. Rapid synthesis of oligomannosides with orthogonally protected monosaccharides. Chem. Commun. 2013, 49, 4265–4267. [Google Scholar] [CrossRef] [PubMed]
- Crich, D.; Smith, M. 1-Benzenesulfinyl piperidine/trifluoromethanesulfonic anhydride: A potent combination of shelf-stable reagents for the low-temperature conversion of thioglycosides to glycosyl triflates and for the formation of diverse glycosidic linkages. J. Am. Chem. Soc. 2001, 123, 9015–9020. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Makinen, K.; Savolainen, J.; Leino, R. Chemistry and biology of oligovalent beta-(1–>2)-linked oligomannosides: New insights into carbohydrate-based adjuvants in immunotherapy. Chemistry 2013, 19, 7961–7974. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Kovac, P. Chemical Synthesis of the Galacturonic Acid Containing Pentasaccharide Antigen of the O-Specific Polysaccharide of Vibrio cholerae O139 and Its Five Fragments. J. Org. Chem. 2016, 81, 6374–6394. [Google Scholar] [CrossRef] [PubMed]
- Gervay, J.; Nguyen, T.N.; Hadd, M.J. Mechanistic studies on the stereoselective formation of glycosyl iodides: First characterization of β-d-glycosyl iodides. Carbohydr. Res. 1997, 300, 119–125. [Google Scholar] [CrossRef]
- Gervay-Hague, J. Taming the Reactivity of Glycosyl Iodides To Achieve Stereoselective Glycosidation. Acc. Chem. Res. 2016, 49, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Hadd, M.J.; Gervay, J. Glycosyl iodides are highly efficient donors under neutral conditions. Carbohydr. Res. 1999, 320, 61–69. [Google Scholar] [CrossRef]
- Schombs, M.; Park, F.E.; Du, W.; Kulkarni, S.S.; Gervay-Hague, J. One-pot syntheses of immunostimulatory glycolipids. J. Org. Chem. 2010, 75, 4891–4898. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Fettinger, J.C.; Gervay-Hague, J. Tandem glycosyl iodide glycosylation and regioselective enzymatic acylation affords 6-O-tetradecanoyl-alpha-d-cholesterylglycosides. J. Org. Chem. 2014, 79, 8447–8452. [Google Scholar] [CrossRef] [PubMed]
- Dabideen, D.R.; Gervay-Hague, J. Unique reactions of glycosyl iodides with oxa- and thiocycloalkane acceptors. Org. Lett. 2004, 6, 973–975. [Google Scholar] [CrossRef] [PubMed]
- El-Badri, M.H.; Willenbring, D.; Tantillo, D.J.; Gervay-Hague, J. Mechanistic studies on the stereoselective formation of beta-mannosides from mannosyl iodides using alpha-deuterium kinetic isotope effects. J. Org. Chem. 2007, 72, 4663–4672. [Google Scholar] [CrossRef] [PubMed]
- El-Badry, M.H.; Gervay-Hague, J. Thermal effect in beta-selective glycosylation reactions using glycosyl iodides. Tetrahedron Lett. 2005, 46, 6727–6728. [Google Scholar] [CrossRef]
- Davis, R.A.; Lin, C.H.; Gervay-Hague, J. Chemoenzymatic synthesis of cholesteryl-6-O-tetradecanoyl-alpha-d-glucopyranoside: A product of host cholesterol efflux promoted by Helicobacter pylori. Chem. Commun. 2012, 48, 9083–9085. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.W.; Davis, R.A.; Hoch, J.A.; Gervay-Hague, J. Two-step functionalization of oligosaccharides using glycosyl iodide and trimethylene oxide and its applications to multivalent glycoconjugates. Chemistry 2014, 20, 6444–6454. [Google Scholar] [CrossRef] [PubMed]
- Bettahi, I.; Dasgupta, G.; Renaudet, O.; Chentoufi, A.A.; Zhang, X.; Carpenter, D.; Yoon, S.; Dumy, P.; BenMohamed, L. Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B cell, CD4+ and CD8+ T cell epitopes. Cancer Immunol. Immunother. 2009, 58, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-Y.; Allan, M.; Adamo, R.; Quinn, D.; Zhai, H.; Wu, G.; Clark, K.; Zhou, J.; Ortiz, S.; Wang, B.; et al. Synthesis of a well-defined glycoconjugate vaccine by a tyrosine-selective conjugation strategy. Chem. Sci. 2013, 4, 3827–3832. [Google Scholar] [CrossRef]
- Stefanetti, G.; Hu, Q.Y.; Usera, A.; Robinson, Z.; Allan, M.; Singh, A.; Imase, H.; Cobb, J.; Zhai, H.; Quinn, D.; et al. Sugar-Protein Connectivity Impacts on the Immunogenicity of Site-Selective Salmonella O-Antigen Glycoconjugate Vaccines. Angew. Chem. Int. Ed. Engl. 2015, 54, 13198–13203. [Google Scholar] [CrossRef] [PubMed]
- Nilo, A.; Passalacqua, I.; Fabbrini, M.; Allan, M.; Usera, A.; Carboni, F.; Brogioni, B.; Pezzicoli, A.; Cobb, J.; Romano, M.R.; et al. Exploring the Effect of Conjugation Site and Chemistry on the Immunogenicity of an anti-Group B Streptococcus Glycoconjugate Vaccine Based on GBS67 Pilus Protein and Type V Polysaccharide. Bioconjug. Chem. 2015, 26, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.; Hu, Q.-Y.; Torosantucci, A.; Crotti, S.; Brogioni, G.; Allan, M.; Chiani, P.; Bromuro, C.; Quinn, D.; Tontini, M.; et al. Deciphering the structure-immunogenicity relationship of anti-Candida glycoconjugate vaccines. Chem. Sci. 2014, 5, 4302–4311. [Google Scholar] [CrossRef]
- Buskas, T.; Thompson, P.; Boons, G.J. Immunotherapy for cancer: Synthetic carbohydrate-based vaccines. Chem. Commun. 2009, 5335–5349. [Google Scholar] [CrossRef] [PubMed]
- Keding, S.J.; Danishefsky, S.J. Prospects for total synthesis: A vision for a totally synthetic vaccine targeting epithelial tumors. Proc. Natl. Acad. Sci. USA 2004, 101, 11937–11942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peri, F. Clustered carbohydrates in synthetic vaccines. Chem. Soc. Rev. 2013, 42, 4543–4556. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragupathi, G.; Coltart, D.M.; Williams, L.J.; Koide, F.; Kagan, E.; Allen, J.; Harris, C.; Glunz, P.W.; Livingston, P.O.; Danishefsky, S.J. On the power of chemical synthesis: Immunological evaluation of models for multiantigenic carbohydrate-based cancer vaccines. Proc. Natl. Acad. Sci. USA 2002, 99, 13699–13704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragupathi, G.; Koide, F.; Livingston, P.O.; Cho, Y.S.; Endo, A.; Wan, Q.; Spassova, M.K.; Keding, S.J.; Allen, J.; Ouerfelli, O.; et al. Preparation and evaluation of unimolecular pentavalent and hexavalent antigenic constructs targeting prostate and breast cancer: A synthetic route to anticancer vaccine candidates. J. Am. Chem. Soc. 2006, 128, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, M.E.; Leroux, J.C.; Castagner, B. Targeting bacterial toxins. Angew. Chem. Int. Ed. Engl. 2012, 51, 4024–4045. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Bundle, D.R. Designing a new antifungal glycoconjugate vaccine. Chem. Soc. Rev. 2013, 42, 4327–4344. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, N.; Maiti, K.; Naresh, K. Multivalent glycoliposomes and micelles to study carbohydrate-protein and carbohydrate-carbohydrate interactions. Chem. Soc. Rev. 2013, 42, 4640–4656. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, S.; Trummer, B.J.; Deng, C.; Wang, A. Carbohydrate microarrays for the recognition of cross-reactive molecular markers of microbes and host cells. Nat. Biotechnol. 2002, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Houseman, B.T.; Mrksich, M. Carbohydrate arrays for the evaluation of protein binding and enzymatic modification. Chem. Biol. 2002, 9, 443–454. [Google Scholar] [CrossRef]
- Fukui, S.; Feizi, T.; Galustian, C.; Lawson, A.M.; Chai, W. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate-protein interactions. Nat. Biotechnol. 2002, 20, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Bryan, M.C.; Plettenburg, O.; Sears, P.; Rabuka, D.; Wacowich-Sgarbi, S.; Wong, C.H. Saccharide display on microtiter plates. Chem. Biol. 2002, 9, 713–720. [Google Scholar] [CrossRef]
- Liang, P.H.; Wu, C.Y.; Greenberg, W.A.; Wong, C.H. Glycan arrays: Biological and medical applications. Curr. Opin. Chem. Biol. 2008, 12, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Song, E.H.; Pohl, N.L. Carbohydrate arrays: Recent developments in fabrication and detection methods with applications. Curr. Opin. Chem. Biol. 2009, 13, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Oyelaran, O.; Gildersleeve, J.C. Glycan arrays: Recent advances and future challenges. Curr. Opin. Chem. Biol. 2009, 13, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Gildersleeve, J.C.; Blixt, O.; Shin, I. Carbohydrate microarrays. Chem. Soc. Rev. 2013, 42, 4310–4326. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Thayer, D.A.; Chang, A.Y.; Best, M.D.; Hoffmann, J.; Head, S.; Wong, C.H. Carbohydrate microarray for profiling the antibodies interacting with Globo H tumor antigen. Proc. Natl. Acad. Sci. USA 2006, 103, 15–20. [Google Scholar] [CrossRef] [PubMed]


















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.S.; Hsieh, H.-W.; Gervay-Hague, J. Anomeric O-Functionalization of Carbohydrates for Chemical Conjugation to Vaccine Constructs. Molecules 2018, 23, 1742. https://doi.org/10.3390/molecules23071742
Park SS, Hsieh H-W, Gervay-Hague J. Anomeric O-Functionalization of Carbohydrates for Chemical Conjugation to Vaccine Constructs. Molecules. 2018; 23(7):1742. https://doi.org/10.3390/molecules23071742
Chicago/Turabian StylePark, Simon S., Hsiao-Wu Hsieh, and Jacquelyn Gervay-Hague. 2018. "Anomeric O-Functionalization of Carbohydrates for Chemical Conjugation to Vaccine Constructs" Molecules 23, no. 7: 1742. https://doi.org/10.3390/molecules23071742
APA StylePark, S. S., Hsieh, H. -W., & Gervay-Hague, J. (2018). Anomeric O-Functionalization of Carbohydrates for Chemical Conjugation to Vaccine Constructs. Molecules, 23(7), 1742. https://doi.org/10.3390/molecules23071742