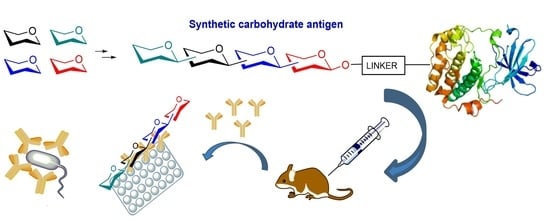
Recent Advances in the Synthesis of Glycoconjugates for Vaccine Development
Abstract
:1. Introduction
2. Shigella
3. Clostridium difficile
3.1. PS-I-Clostridium difficile
3.2. PS-II-Clostridium difficile
4. Burkholderia pseudomallei
5. Brucella
6. Haemophilus influenzae Type b (Hib)
7. Streptococcus pneumoniae
7.1. S. pneumoniae Serotype 1
7.2. S. pneumoniae Serotype 2
7.3. S. pneumoniae Serotype 3
7.4. S. pneumoniae Serotype 4
7.5. S. pneumoniae Serotype 5
7.6. S. pneumoniae Serotype 8
7.7. S. pneumoniae Serotypes 14 and 19F
8. Group A Streptococcus
9. Group B Streptococcus
10. Salmonella Typhi
11. Pseudomonas aeruginosa
12. Neisseria meningitidis
12.1. N. meningitidis Serogroup A (MenA)
12.2. N. meningitidis Serogroup C (MenC)
12.3. N. meningitidis Serogroup W (MenW)
12.4. N. meningitidis Serogroup X (MenX)
13. Mycobacterium tuberculosis
14. Fungal Infections
14.1. Candida albicans
14.2. Aspergillus fumigatus
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vaccination Greatly Reduces Disease, Disability, Death and Inequity Worldwide. Available online: http://www.who.int/bulletin/volumes/86/2/07-040089/en/ (accessed on 28 February 2018).
- Bentley, S.D.; Aanensen, D.M.; Mavroidi, A.; Saunders, D.; Rabbinowitsch, E.; Collins, M.; Donohoe, K.; Harris, D.; Murphy, L.; Quail, M.A.; et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006, 2, e31. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A novel mechanism for glycoconjugate vaccine activation of the adaptive immune system. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avci, F.Y.; Kasper, D.L. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 2010, 28, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Adamo, R. Recent mechanistic insights on glycoconjugate vaccines and future perspectives. ACS Chem. Biol. 2013, 8, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.; Nilo, A.; Castagner, B.; Boutureira, O.; Berti, F.; Bernardes, G.J.L. Synthetically defined glycoprotein vaccines: Current status and future directions. Chem. Sci. 2013, 4, 2995–3008. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.W.; Oaks, E.V. Inactivated and subunit vaccines to prevent shigellosis. Expert Rev. Vaccines 2009, 8, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Wierzba, T.; Walker, R.I. Status of vaccine research and development for shigella. Vaccine 2016, 34, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Verez-Bencomo, V.; Fernández-Santana, V.; Hardy, E.; Toledo, M.E.; Rodríguez, M.C.; Heynngnezz, L.; Rodriguez, A.; Baly, A.; Herrera, L.; Izquierdo, M.; et al. A synthetic conjugate polysaccharide vaccine against haemophilus influenzae type b. Science 2004, 305, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Astronomo, R.D.; Burton, D.R. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 2010, 9, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Poletti, L.; Lay, L. Carbohydrates and immunology: Synthetic oligosaccharide antigens for vaccine formulation. Eur. J. Org. Chem. 2011, 2011, 5723–5777. [Google Scholar] [CrossRef]
- The Symbolic Representation of Monosaccharides in the Age of Glycobiology. Available online: https://glycopedia.eu/References-102 (accessed on 4 April 2018).
- Pozsgay, V. Synthesis of glycoconjugate vaccines against shigella dysenteriae type 1. J. Org. Chem. 1998, 63, 5983–5999. [Google Scholar] [CrossRef] [PubMed]
- Pozsgay, V. Synthetic shigella vaccines: A carbohydrate–protein conjugate with totally synthetic hexadecasaccharide haptens. Angew. Chem. Int. Ed. 1998, 37, 138–142. [Google Scholar] [CrossRef]
- Pozsgay, V.; Kubler-Kielb, J.; Schneerson, R.; Robbins, J.B. Effect of the nonreducing end of shigella dysenteriae type 1 o-specific oligosaccharides on their immunogenicity as conjugates in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 14478–14482. [Google Scholar] [CrossRef] [PubMed]
- Bélot, F.; Wright, K.; Costachel, C.; Phalipon, A.; Mulard, L.A. Blockwise approach to fragments of the o-specific polysaccharide of shigella flexneri serotype 2a: Convergent synthesis of a decasaccharide representative of a dimer of the branched repeating unit1. J. Org. Chem. 2004, 69, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Belot, F.; Guerreiro, C.; Baleux, F.; Mulard, L.A. Synthesis of two linear padre conjugates bearing a deca- or pentadecasaccharide b epitope as potential synthetic vaccines against shigella flexneri serotype 2a infection. Chem. Eur. J. 2005, 11, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Van der Put, R.M.; Kim, T.H.; Guerreiro, C.; Thouron, F.; Hoogerhout, P.; Sansonetti, P.J.; Westdijk, J.; Stork, M.; Phalipon, A.; Mulard, L.A. A synthetic carbohydrate conjugate vaccine candidate against shigellosis: Improved bioconjugation and impact of alum on immunogenicity. Bioconjug. Chem. 2016, 27, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Phalipon, A.; Costachel, C.; Grandjean, C.; Thuizat, A.; Guerreiro, C.; Tanguy, M.; Nato, F.; Vulliez-Le Normand, B.; Belot, F.; Wright, K.; et al. Characterization of functional oligosaccharide mimics of the shigella flexneri serotype 2a o-antigen: Implications for the development of a chemically defined glycoconjugate vaccine. J. Immunol. 2006, 176, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Phalipon, A.; Tanguy, M.; Grandjean, C.; Guerreiro, C.; Belot, F.; Cohen, D.; Sansonetti, P.J.; Mulard, L.A. A synthetic carbohydrate-protein conjugate vaccine candidate against shigella flexneri 2a infection. J. Immunol. 2009, 182, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Chassagne, P.; Theillet, F.X.; Guerreiro, C.; Thouron, F.; Nato, F.; Delepierre, M.; Sansonetti, P.J.; Phalipon, A.; Mulard, L.A. Non-stoichiometric o-acetylation of shigella flexneri 2a o-specific polysaccharide: Synthesis and antigenicity. Org. Biomol. Chem. 2014, 12, 4218–4232. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; De Ricco, R.; Rappuoli, R. Role of o-acetylation in the immunogenicity of bacterial polysaccharide vaccines. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Hlozek, J.; Kuttel, M.M.; Ravenscroft, N. Conformations of neisseria meningitidis serogroup a and x polysaccharides: The effects of chain length and o-acetylation. Carbohydr. Res. 2018, 465, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mulard, L.A. Bacterial polysaccharides as major surface antigens: Interest in o-acetyl substitutions. In Carbohydrate Chemistry: Chemical and Biological Approaches Volume 43; RSC Publishing: London, UK, 2018; Volume 43, pp. 71–103. [Google Scholar]
- Rebeaud, F.; Bachmann, M.F. Immunization strategies for clostridium difficile infections. Expert Rev. Vaccines 2012, 11, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Bauer, M.P.; Baines, S.D.; Corver, J.; Fawley, W.N.; Goorhuis, B.; Kuijper, E.J.; Wilcox, M.H. The changing epidemiology of clostridium difficile infections. Clin. Microbiol. Rev. 2010, 23, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Péchiné, S.; Janoir, C.; Collignon, A. Emerging monoclonal antibodies against clostridium difficile infection. Expert Opin. Biol. Ther. 2017, 17, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; Pea, F.; Masucci, L.; Posteraro, P.; Sanguinetti, M. Actoxumab + bezlotoxumab combination: What promise for clostridium difficile treatment? Expert Opin. Biol. Ther. 2018, 18, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.E.; Broecker, F.; Oberli, M.A.; Komor, J.; Mattner, J.; Anish, C.; Seeberger, P.H. Immunological evaluation of a synthetic clostridium difficile oligosaccharide conjugate vaccine candidate and identification of a minimal epitope. J. Am. Chem. Soc. 2013, 135, 9713–9722. [Google Scholar] [CrossRef] [PubMed]
- Broecker, F.; Hanske, J.; Martin, C.E.; Baek, J.Y.; Wahlbrink, A.; Wojcik, F.; Hartmann, L.; Rademacher, C.; Anish, C.; Seeberger, P.H. Multivalent display of minimal clostridium difficile glycan epitopes mimics antigenic properties of larger glycans. Nat. Commun. 2016, 7, 11224. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, F.; O’Brien Alexander, G.; Götze, S.; Seeberger Peter, H.; Hartmann, L. Synthesis of carbohydrate-functionalised sequence-defined oligo(amidoamine)s by photochemical thiolene coupling in a continuous flow reactor. Chem. Eur. J. 2013, 19, 3090–3098. [Google Scholar] [CrossRef] [PubMed]
- Oberli, M.A.; Hecht, M.-L.; Bindschädler, P.; Adibekian, A.; Adam, T.; Seeberger, P.H. A possible oligosaccharide-conjugate vaccine candidate for clostridium difficile is antigenic and immunogenic. Chem. Biol. 2011, 18, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Danieli, E.; Lay, L.; Proietti, D.; Berti, F.; Costantino, P.; Adamo, R. First synthesis of c. Difficile ps-ii cell wall polysaccharide repeating unit. Org. Lett. 2011, 13, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.; Romano, M.R.; Berti, F.; Leuzzi, R.; Tontini, M.; Danieli, E.; Cappelletti, E.; Cakici, O.S.; Swennen, E.; Pinto, V.; et al. Phosphorylation of the synthetic hexasaccharide repeating unit is essential for the induction of antibodies to clostridium difficile psii cell wall polysaccharide. ACS Chem. Biol. 2012, 7, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Meumann, E.M.; Cheng, A.C.; Ward, L.; Currie, B.J. Clinical features and epidemiology of melioidosis pneumonia: Results from a 21-year study and review of the literature. Clin. Infect. Dis. 2012, 54, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Dance, D. Treatment and prophylaxis of melioidosis. Int. J. Antimicrob. Agents 2014, 43, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.E.; Christ, W.J.; George, A.J.; Stokes, M.G.; Lohman, G.J.; Guo, Y.; Jones, M.; Titball, R.W.; Atkins, T.P.; Campbell, A.S.; et al. Protection against experimental melioidosis with a synthetic manno-heptopyranose hexasaccharide glycoconjugate. Bioconjug. Chem. 2016, 27, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Conejero, L.; De Reynal, M.; Easton, A.; Bancroft, G.J.; Titball, R.W. Development of vaccines against burkholderia pseudomallei. Front. Microbiol. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.B.; Dow, S.W. Development of burkholderia mallei and pseudomallei vaccines. Front. Cell. Infect. Microbiol. 2013, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Burtnick, M.N.; Shaffer, T.L.; Ross, B.N.; Muruato, L.A.; Sbrana, E.; DeShazer, D.; Torres, A.G.; Brett, P.J. Development of subunit vaccines that provide high-level protection and sterilizing immunity against acute inhalational melioidosis. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef] [PubMed]
- Reckseidler, S.L.; DeShazer, D.; Sokol, P.A.; Woods, D.E. Detection of bacterial virulence genes by subtractive hybridization: Identification of capsular polysaccharide of burkholderia pseudomallei as a major virulence determinant. Infect. Immun. 2001, 69, 34–44. [Google Scholar] [CrossRef] [PubMed]
- DeShazer, D.; Waag, D.M.; Fritz, D.L.; Woods, D.E. Identification of a burkholderia mallei polysaccharide gene cluster by subtractive hybridization and demonstration that the encoded capsule is an essential virulence determinant. Microb. Pathog. 2001, 30, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Boschiroli, M.-L.; Foulongne, V.; O’Callaghan, D. Brucellosis: A worldwide zoonosis. Curr. Opin. Microbiol. 2001, 4, 58–64. [Google Scholar] [CrossRef]
- Mantur, B.; Akki, A.; Mangalgi, S.S.; Patil, S.; Gobbur, R.; Peerapur, B. Childhood brucellosis—A microbiological, epidemiological and clinical study. J. Trop. Pediatr. 2004, 50, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Mantur, B.G.; Biradar, M.S.; Bidri, R.C.; Mulimani, M.S.; Veerappa, K.; Kariholu, P.; Patil, S.B.; Mangalgi, S.S. Protean clinical manifestations and diagnostic challenges of human brucellosis in adults: 16 years’ experience in an endemic area. J. Med. Microbiol. 2006, 55, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Cloeckaert, A.; Moriyón, I. Brucella evolution and taxonomy. Vet. Microbiol. 2002, 90, 209–227. [Google Scholar] [CrossRef]
- Cloeckaert, A.; Grayon, M.; Grépinet, O.; Boumedine, K.S. Classification of brucella strains isolated from marine mammals by infrequent restriction site-PCR and development of specific PCR identification tests. Microb. Infect. 2003, 5, 593–602. [Google Scholar] [CrossRef]
- Roth, F.; Zinsstag, J.; Orkhon, D.; Chimed-Ochir, G.; Hutton, G.; Cosivi, O.; Carrin, G.; Otte, J. Human health benefits from livestock vaccination for brucellosis: Case study. Bull. World Health Organ. 2003, 81, 867–876. [Google Scholar] [PubMed]
- Bundle, D.R.; McGiven, J. Brucellosis: Improved diagnostics and vaccine insights from synthetic glycans. Acc. Chem. Res. 2017, 50, 2958–2967. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, M.S.; Bundle, D.R.; Ganesh, N.V.; Guiard, J.; Cloeckaert, A. Monoclonal antibody-defined specific c epitope of brucella o-polysaccharide revisited. Clin. Vaccine Immunol. 2015, 22, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Smits, H.L.; Basahi, M.A.; Díaz, R.; Marrodan, T.; Douglas, J.T.; Rocha, A.; Veerman, J.; Zheludkov, M.M.; Witte, O.W.M.; de Jong, J.; et al. Development and evaluation of a rapid dipstick assay for serodiagnosis of acute human brucellosis. J. Clin. Microbiol. 1999, 37, 4179–4182. [Google Scholar] [PubMed]
- Clavijo, E.; Díaz, R.; Anguita, A.; García, A.; Pinedo, A.; Smits, H.L. Comparison of a dipstick assay for detection of brucella-specific immunoglobulin m antibodies with other tests for serodiagnosis of human brucellosis. Clin. Diagn. Lab. Immunol. 2003, 10, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Kubler-Kielb, J.; Vinogradov, E. Reinvestigation of the structure of brucella o-antigens. Carbohydr. Res. 2013, 378, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Guiard, J.; Paszkiewicz, E.; Sadowska, J.; Bundle, D.R. Design and synthesis of a universal antigen to detect brucellosis. Angew. Chem. Int. Ed. Engl. 2013, 52, 7181–7185. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K. Diagnosis of brucellosis by serology. Vet. Microbiol. 2002, 90, 447–459. [Google Scholar] [CrossRef]
- Ganesh, N.V.; Sadowska, J.M.; Sarkar, S.; Howells, L.; McGiven, J.; Bundle, D.R. Molecular recognition of brucella a and m antigens dissected by synthetic oligosaccharide glycoconjugates leads to a disaccharide diagnostic for brucellosis. J. Am. Chem. Soc. 2014, 136, 16260–16269. [Google Scholar] [CrossRef] [PubMed]
- McGiven, J.; Howells, L.; Duncombe, L.; Stack, J.; Ganesh, N.V.; Guiard, J.; Bundle, D.R. Improved serodiagnosis of bovine brucellosis by novel synthetic oligosaccharide antigens representing the capping m epitope elements of brucella o-polysaccharide. J. Clin. Microbiol. 2015, 53, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.S.; Duncombe, L.; Ganesh, N.V.; Sarkar, S.; Howells, L.; Hogarth, P.J.; Bundle, D.R.; McGiven, J. Novel solutions for vaccines and diagnostics to combat brucellosis. ACS Cent. Sci. 2017, 3, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Murphy, T.F. Haemophilus influenzae infections in the h. Influenzae type b conjugate vaccine era. J. Clin. Microbiol. 2011, 49, 3728–3732. [Google Scholar] [CrossRef] [PubMed]
- Peltola, H.; Käythy, H.; Sivonen, A.; Mäkelä, P.H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: A double-blind field study of 100,000 vaccinees 3 months to 5 years of age in finland. Pediatrics 1977, 60, 730–737. [Google Scholar] [PubMed]
- Shivatare, S.S.; Wu, C.-Y. Carbohydrate-based antibacterial vaccines: Current progress and future outlook. In Carbohydrate Chemistry: State of the Art and Challenges for Drug Development: An Overview on Structure, Biological Roles, Synthetic Methods and Application as Therapeutics; World Scientific Publishers: Singapore, 2015; pp. 321–356. [Google Scholar]
- Fernández-Santana, V.; Cardoso, F.; Rodriguez, A.; Carmenate, T.; Peña, L.; Valdés, Y.; Hardy, E.; Mawas, F.; Heynngnezz, L.; Rodríguez, M.C.; et al. Antigenicity and immunogenicity of a synthetic oligosaccharide-protein conjugate vaccine against haemophilus influenzae type b. Infect. Immun. 2004, 72, 7115–7123. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Geissner, A.; Rathwell, D.C.K.; Meierhofer, D.; Pereira, C.L.; Seeberger, P.H. A modular synthetic route to size-defined immunogenic haemophilus influenzae b antigens is key to the identification of an octasaccharide lead vaccine candidate. Chem. Sci. 2018, 9, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, E.N.; Oliveira, M.L.; Carvalho, E.; Ho, P.L. Serotype-independent pneumococcal vaccines. Cell. Mol. Life Sci. 2013, 70, 3303–3326. [Google Scholar] [CrossRef] [PubMed]
- Trotter, C.L.; Waight, P.; Andrews, N.J.; Slack, M.; Efstratiou, A.; George, R.; Miller, E. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: England and wales, 1996–2006. J. Infect. 2010, 60, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Pelton, S.I.; Loughlin, A.M.; Marchant, C.D. Seven valent pneumococcal conjugate vaccine immunization in two boston communities: Changes in serotypes and antimicrobial susceptibility among streptococcus pneumoniae isolates. Pediatr. Infect. Dis. J. 2004, 23, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H.; Pereira, C.L.; Govindan, S. Total synthesis of a streptococcus pneumoniae serotype 12f cps repeating unit hexasaccharide. Beilstein J. Org. Chem. 2017, 13, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Perciani, C.T.; Barazzone, G.C.; Goulart, C.; Carvalho, E.; Cabrera-Crespo, J.; Gonçalves, V.M.; Leite, L.C.C.; Tanizaki, M.M. Conjugation of polysaccharide 6b from streptococcus pneumoniae with pneumococcal surface protein a: Pspa conformation and its effect on the immune response. Clin. Vaccine Immunol. 2013, 20, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cui, L.; Lipinski, T.; Bundle, D.R. Synthesis of monomeric and dimeric repeating units of the zwitterionic type 1 capsular polysaccharide from streptococcus pneumoniae. Chem. Eur. J. 2010, 16, 3476–3488. [Google Scholar] [CrossRef] [PubMed]
- Christina, A.E.; van den Bos, L.J.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Galacturonic acid lactones in the synthesis of all trisaccharide repeating units of the zwitterionic polysaccharide sp1. J. Org. Chem. 2011, 76, 1692–1706. [Google Scholar] [CrossRef] [PubMed]
- Schumann, B.; Pragani, R.; Anish, C.; Pereira, C.L.; Seeberger, P.H. Synthesis of conjugation-ready zwitterionic oligosaccharides by chemoselective thioglycoside activation. Chem. Sci. 2014, 5, 1992–2002. [Google Scholar] [CrossRef]
- Schumann, B.; Reppe, K.; Kaplonek, P.; Wahlbrink, A.; Anish, C.; Witzenrath, M.; Pereira, C.L.; Seeberger, P.H. Development of an efficacious, semisynthetic glycoconjugate vaccine candidate against streptococcus pneumoniae serotype 1. ACS Cent. Sci. 2018, 4, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Singh, M.; Das, R.R.; Jindal, I.; Agarwal, A.; Thumburu, K.K.; Kumar, A.; Chauhan, A. Distribution of serotypes, vaccine coverage, and antimicrobial susceptibility pattern of streptococcus pneumoniae in children living in saarc countries: A systematic review. PLoS ONE 2014, 9, e108617. [Google Scholar] [CrossRef] [PubMed]
- Gaensbauer, J.T.; Asturias, E.J.; Soto, M.; Holt, E.; Olson, D.; Halsey, N.A.; Guatemala Pediatric Bacterial Surveillance Working Group. Pediatric invasive pneumococcal disease in guatemala city: Importance of serotype 2. Pediatr. Infect. Dis. J. 2016, 35, e139–e143. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.-E.; Lindberg, B.; Andersson, M.; Lindquist, U.; Henrichsen, J. Structural studies of the capsular polysaccharide from streptococcus pneumoniae type 2, a reinvestigation. Carbohydr. Res. 1988, 182, 111–117. [Google Scholar] [CrossRef]
- Emmadi, M.; Khan, N.; Lykke, L.; Reppe, K.; Parameswarappa, S.G.; Lisboa, M.P.; Wienhold, S.M.; Witzenrath, M.; Pereira, C.L.; Seeberger, P.H. A streptococcus pneumoniae type 2 oligosaccharide glycoconjugate elicits opsonic antibodies and is protective in an animal model of invasive pneumococcal disease. J. Am. Chem. Soc. 2017, 139, 14783–14791. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.; Kriz, P.; Feron, C.; Di-Paolo, E.; Henckaerts, I.; Miseur, A.; Wauters, D.; Prymula, R.; Schuerman, L. Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine 2009, 27, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.; Borrow, R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev. Vaccines 2011, 10, 307–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truck, J.; Thompson, A.; Morales-Aza, B.; Clutterbuck, E.A.; Voysey, M.; Clarke, E.; Snape, M.D.; Kelly, D.F.; Finn, A.; Pollard, A.J. Memory b cell response to a PCV-13 booster in 3.5year old children primed with either PCV-7 or PCV-13. Vaccine 2017, 35, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Benaissa-Trouw, B.; Lefeber, D.J.; Kamerling, J.P.; Vliegenthart, J.F.; Kraaijeveld, K.; Snippe, H. Synthetic polysaccharide type 3-related di-, tri-, and tetrasaccharide-CRM(197) conjugates induce protection against streptococcus pneumoniae type 3 in mice. Infect. Immun. 2001, 69, 4698–4701. [Google Scholar] [CrossRef] [PubMed]
- Parameswarappa, S.G.; Reppe, K.; Geissner, A.; Menova, P.; Govindan, S.; Calow, A.D.J.; Wahlbrink, A.; Weishaupt, M.W.; Monnanda, B.P.; Bell, R.L.; et al. A semi-synthetic oligosaccharide conjugate vaccine candidate confers protection against streptococcus pneumoniae serotype 3 infection. Cell Chem. Biol. 2016, 23, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Feng, S.; Qiao, Y.; Guo, Z.; Gu, G. Synthesis and immunological studies of oligosaccharides that consist of the repeating unit of streptococcus pneumoniae serotype 3 capsular polysaccharide. Chem. Eur. J. 2018, 24, 8205–8216. [Google Scholar] [CrossRef]
- Wang, L.; Feng, S.; An, L.; Gu, G.; Guo, Z. Synthetic and immunological studies of mycobacterial lipoarabinomannan oligosaccharides and their protein conjugates. J. Org. Chem. 2015, 80, 10060–10075. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.L.; Geissner, A.; Anish, C.; Seeberger, P.H. Chemical synthesis elucidates the immunological importance of a pyruvate modification in the capsular polysaccharide of streptococcus pneumoniae serotype 4. Angew. Chem. Int. Ed. 2015, 54, 10016–10019. [Google Scholar] [CrossRef] [PubMed]
- Geissner, A.; Pereira, C.L.; Leddermann, M.; Anish, C.; Seeberger, P.H. Deciphering antigenic determinants of streptococcus pneumoniae serotype 4 capsular polysaccharide using synthetic oligosaccharides. ACS Chem. Biol. 2016, 11, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Sucher, A.J.; Chahine, E.B.; Nelson, M.; Sucher, B.J. Prevnar 13, the new 13-valent pneumococcal conjugate vaccine. Ann. Pharmacother. 2011, 45, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.-E.; Lindberg, B.; Lindquist, U. Structural studies of the capsular polysaccharide from streptococcus pneumoniae type 5. Carbohydr. Res. 1985, 140, 101–110. [Google Scholar] [CrossRef]
- Mistretta, N.; Danve, E.; Moreau, M. Conjugates Obtained by Reductive Amination of the Pneumococcus Serotype 5 Capsular Polysaccharide. US Patent 7812006B2, 12 October 2010. [Google Scholar]
- Lisboa, M.P.; Khan, N.; Martin, C.; Xu, F.-F.; Reppe, K.; Geissner, A.; Govindan, S.; Witzenrath, M.; Pereira, C.L.; Seeberger, P.H. Semisynthetic glycoconjugate vaccine candidate against streptococcus pneumoniae serotype 5. Proc. Natl. Acad. Sci. USA 2017, 114, 11063–11068. [Google Scholar] [CrossRef] [PubMed]
- Ardanuy, C.; de la Campa, A.G.; García, E.; Fenoll, A.; Calatayud, L.; Cercenado, E.; Pérez-Trallero, E.; Bouza, E.; Liñares, J. Spread of streptococcus pneumoniae serotype 8-ST63 multidrug-resistant recombinant clone, spain. Emerg. Infect. Dis. 2014, 20, 1848–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, J.C.; Cercenado, E.; Marín, M.; Ramos, B.; Ardanuy, C.; Rodríguez-Avial, I.; Bouza, E. Multidrug-resistant pneumococci (serotype 8) causing invasive disease in hiv+ patients. Clin. Microbiol. Infect. 2011, 17, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Schumann, B.; Hahm, H.S.; Parameswarappa, S.G.; Reppe, K.; Wahlbrink, A.; Govindan, S.; Kaplonek, P.; Pirofski, L.-A.; Witzenrath, M.; Anish, C.; et al. A semisynthetic streptococcus pneumoniae serotype 8 glycoconjugate vaccine. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Pirofski, L.-A. Characterization of gene use and efficacy of mouse monoclonal antibodies to streptococcus pneumoniae serotype 8. Clin. Vaccine Immunol. 2011, 18, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Hao, T.; Ou, S.; Hu, C.; Chen, L. Applications and perspectives of nanomaterials in novel vaccine development. MedChemComm 2018, 9, 226–238. [Google Scholar] [CrossRef]
- Compostella, F.; Pitirollo, O.; Silvestri, A.; Polito, L. Glyco-gold nanoparticles: Synthesis and applications. Beilstein J. Org. Chem. 2017, 13, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Safari, D.; Marradi, M.; Chiodo, F.; Th Dekker, H.A.; Shan, Y.; Adamo, R.; Oscarson, S.; Rijkers, G.T.; Lahmann, M.; Kamerling, J.P. Gold nanoparticles as carriers for a synthetic streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine 2012, 7, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, B.; Lonngren, J.; Powell, D.A. Structural studies on the specific type-14 pneumococcal polysaccharide. Carbohydr. Res. 1977, 58, 177–186. [Google Scholar] [CrossRef]
- Vetro, M.; Safari, D.; Fallarini, S.; Salsabila, K.; Lahmann, M.; Penadés, S.; Lay, L.; Marradi, M.; Compostella, F. Preparation and immunogenicity of gold glyco-nanoparticles as antipneumococcal vaccine model. Nanomedicine 2017, 12, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Mawas, F.; Niggemann, J.; Jones, C.; Corbel, M.J.; Kamerling, J.P.; Vliegenthart, J.F.G. Immunogenicity in a mouse model of a conjugate vaccine made with a synthetic single repeating unit of type 14 pneumococcal polysaccharide coupled to CRM197. Infect. Immun. 2002, 70, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Safari, D.; Dekker, H.A.; Joosten, J.A.; Michalik, D.; de Souza, A.C.; Adamo, R.; Lahmann, M.; Sundgren, A.; Oscarson, S.; Kamerling, J.P. Identification of the smallest structure capable of evoking opsonophagocytic antibodies against streptococcus pneumoniae type 14. Infect. Immun. 2008, 76, 4615–4623. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, E.; Khitri, M.; Lay, L.; Nicotra, F.; Panza, L.; Russo, G. Capsular polysaccharide of streptococcus pneumoniae type 19f: Synthesis of the repeating unit. Carbohydr. Res. 1998, 311, 171–181. [Google Scholar] [CrossRef]
- Legnani, L.; Ronchi, S.; Fallarini, S.; Lombardi, G.; Campo, F.; Panza, L.; Lay, L.; Poletti, L.; Toma, L.; Ronchetti, F.; et al. Synthesis, molecular dynamics simulations, and biology of a carba-analogue of the trisaccharide repeating unit of streptococcus pneumoniae19f capsular polysaccharide. Org. Biomol. Chem. 2009, 7, 4428–4436. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.L.; Nizet, V. Disease manifestations and pathogenic mechanisms of group a streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef] [PubMed]
- Kabanova, A.; Margarit, I.; Berti, F.; Romano, M.R.; Grandi, G.; Bensi, G.; Chiarot, E.; Proietti, D.; Swennen, E.; Cappelletti, E.; et al. Evaluation of a group a streptococcus synthetic oligosaccharide as vaccine candidate. Vaccine 2010, 29, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Van Sorge, N.M.; Cole, J.N.; Kuipers, K.; Henningham, A.; Aziz, R.K.; Kasirer-Friede, A.; Lin, L.; Berends, E.T.M.; Davies, M.R.; Dougan, G.; et al. The classical lancefield antigen of group a streptococcus is a virulence determinant with implications for vaccine design. Cell Host Microbe 2014, 15, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E.; Hemric, M.E.; Ward, K.; Cunningham, M.W. Cytotoxic mab from rheumatic carditis recognizes heart valves and laminin. J. Clin. Investig. 2000, 106, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kirvan, C.A.; Swedo, S.E.; Heuser, J.S.; Cunningham, M.W. Mimicry and autoantibody-mediated neuronal cell signaling in sydenham chorea. Nat. Med. 2003, 9, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Malkiel, S.; Liao, L.; Cunningham, M.W.; Diamond, B. T-cell-dependent antibody response to the dominant epitope of streptococcal polysaccharide, n-acetyl-glucosamine, is cross-reactive with cardiac myosin. Infect. Immun. 2000, 68, 5803–5808. [Google Scholar] [CrossRef] [PubMed]
- Henningham, A.; Davies, M.R.; Uchiyama, S.; van Sorge, N.M.; Lund, S.; Chen, K.T.; Walker, M.J.; Cole, J.N.; Nizet, V. Virulence role of the glcnac side chain of the lancefield cell wall carbohydrate antigen in non-m1-serotype group a streptococcus. MBio 2018, 9. [Google Scholar] [CrossRef]
- Auzanneau, F.I.; Borrelli, S.; Pinto, B.M. Synthesis and immunological activity of an oligosaccharide-conjugate as a vaccine candidate against group a streptococcus. Bioorg. Med. Chem. Lett. 2013, 23, 6038–6042. [Google Scholar] [CrossRef] [PubMed]
- Reimer, K.B.; Gidney, M.A.J.; Bundle, D.R.; Pinto, B.M. Immunochemical characterization of polyclonal and monoclonal streptococcus group a antibodies by chemically defined glycoconjugates and synthetic oligosaccharides. Carbohydr. Res. 1992, 232, 131–142. [Google Scholar] [CrossRef]
- Johnson, M.A.; Pinto, B.M. Saturation transfer difference 1d-tocsy experiments to map the topography of oligosaccharides recognized by a monoclonal antibody directed against the cell-wall polysaccharide of group a streptococcus. J. Am. Chem. Soc. 2002, 124, 15368–15374. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Rench, M.A.; Edwards, M.S.; Carpenter, R.J.; Hays, B.M.; Kasper, D.L. Immunization of pregnant women with a polysaccharide vaccine of group b streptococcus. N. Engl. J. Med. 1988, 319, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Schuchat, A. Perinatal group b streptococcal disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Skoff, T.H.; Farley, M.M.; Petit, S.; Craig, A.S.; Schaffner, W.; Gershman, K.; Harrison, L.H.; Lynfield, R.; Mohle-Boetani, J.; Zansky, S. Increasing burden of invasive group b streptococcal disease in nonpregnant adults, 1990–2007. Clin. Infect. Dis. 2009, 49, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, A.; Rinaudo, C.D.; Maione, D. Group b streptococcus vaccine: State of the art. Ther. Adv. Vaccines 2015, 3, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.K.; Liao, G.; Mondal, M.A.; Guo, Z. Chemical synthesis of the repeating unit of type ia group b streptococcus capsular polysaccharide. Org. Lett. 2015, 17, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, Z. Chemical synthesis of the repeating unit of type v group b streptococcus capsular polysaccharide. Org. Lett. 2016, 18, 5552–5555. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhang, H.; Li, Y.; Gu, G.; Cai, F.; Guo, Z.; Gao, J. Chemical synthesis of the repeating unit of type ii group b streptococcus capsular polysaccharide. J. Org. Chem. 2018, 83, 5920–5930. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Rench, M.A.; McInnes, P. Immunization of pregnant women with group b streptococcal type iii capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine 2003, 21, 3468–3472. [Google Scholar] [CrossRef]
- Demchenko, A.V.; Boons, G.-J. A highly convergent synthesis of a complex oligosaccharide derived from group b type iii streptococcus. J. Org. Chem. 2001, 66, 2547–2554. [Google Scholar] [CrossRef] [PubMed]
- Pozsgay, V.; Brisson, J.-R.; Jennings, H.J. Synthesis of a tri-and a tetra-saccharide fragment of the capsular polysaccharide of type iii group b streptococcus. Carbohydr. Res. 1990, 205, 133–146. [Google Scholar] [CrossRef]
- Demchenko, A.V.; Boons, G.-J. A novel and versatile glycosyl donor for the preparation of glycosides of n-acetylneuraminic acid. Tetrahedron Lett. 1998, 39, 3065–3068. [Google Scholar] [CrossRef]
- Demchenko, A.; Boons, G.-J. A highly convergent synthesis of a hexasaccharide derived from the oligosaccharide of group b type iii streptococcus. Tetrahedron Lett. 1997, 38, 1629–1632. [Google Scholar] [CrossRef]
- Jennings, H.J.; Lugowski, C.; Kasper, D.L. Conformational aspects critical to the immunospecificity of the type iii group b streptococcal polysaccharide. Biochemistry 1981, 20, 4511–4518. [Google Scholar] [CrossRef] [PubMed]
- González-Outeiriño, J.; Kadirvelraj, R.; Woods, R.J. Structural elucidation of type iii group b streptococcus capsular polysaccharide using molecular dynamics simulations: The role of sialic acid. Carbohydr. Res. 2005, 340, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, V.; Carboni, F.; Oldrini, D.; De Ricco, R.; Donadio, N.; Ros, I.M.Y.; Berti, F.; Adamo, R. Synthesis of group b streptococcus type iii polysaccharide fragments for evaluation of their interactions with monoclonal antibodies. Pure Appl. Chem. 2017, 89, 855–875. [Google Scholar] [CrossRef]
- Weekly Epidemiological Record. 2018, 93, 153–172. Available online: http://www.who.int/wer/2018/wer9313/en/ (accessed on 27 May 2018).
- Britto, C.; Pollard, A.J.; Voysey, M.; Blohmke, C.J. An appraisal of the clinical features of pediatric enteric fever: Systematic review and meta-analysis of the age-stratified disease occurrence. Clin. Infect. Dis. 2017, 64, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Wijedoru, L.; Mallett, S.; Parry, C.M. Rapid diagnostic tests for typhoid and paratyphoid (enteric) fever. Cochrane Database Syst. Rev. 2017, CD008892. [Google Scholar] [CrossRef] [PubMed]
- Olopoenia, L.A.; King, A.L. Widal agglutination test—100 years later: Still plagued by controversy. Postgrad. Med. J. 2000, 76, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Sjölund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Qadri, A. Vi polysaccharide of salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc. Natl. Acad. Sci. USA 2004, 101, 17492–17497. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, S.K.; Dutta, D.; Parween, F.; Qadri, A. The virulence polysaccharide vi released by salmonella typhi targets membrane prohibitin to inhibit t-cell activation. J. Infect. Dis. 2014, 210, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Virlogeux-Payant, I.; Popoff, M.Y. The vi antigen of salmonella typhi. Bull. Inst. Pasteur 1996, 94, 237–250. [Google Scholar] [CrossRef]
- Felix, A.; Pitt, R.M. The vi antigens of various salmonella types. Br. J. Exp. Pathol. 1936, 17, 81–86. [Google Scholar]
- Hu, X.; Chen, Z.; Xiong, K.; Wang, J.; Rao, X.; Cong, Y. Vi capsular polysaccharide: Synthesis, virulence, and application. Crit. Rev. Microbiol. 2017, 43, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Ermin, S.; Petr, D.; Marian, H. Role of salmonella typhi vi antigen and secretory systems on immune response. Curr. Pharm. Des. 2016, 22, 6251–6260. [Google Scholar] [CrossRef]
- Szu, S.C.; Li, X.R.; Stone, A.L.; Robbins, J.B. Relation between structure and immunologic properties of the vi capsular polysaccharide. Infect. Immun. 1991, 59, 4555–4561. [Google Scholar] [PubMed]
- Kiow Shi-Shun, L.; Mallet, J.-M.; Moreau, M.; Sinaÿ, P. Synthèse d’oligomères du polysaccharide capsulaire de salmonella typhi, bactérie à l’origine de la fièvre typhoïde. Tetrahedron 1999, 55, 14043–14068. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, J.; Zheng, X.J.; Tai, G.; Ye, X.S. A highly alpha-stereoselective synthesis of oligosaccharide fragments of the vi antigen from salmonella typhi and their antigenic activities. Chem. Eur. J. 2011, 17, 14518–14526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-L.; Yang, L.; Zhu, J.; Wei, M.; Yan, W.; Xiong, D.-C.; Ye, X.-S. Synthesis and antigenic evaluation of oligosaccharide mimics of vi antigen from salmonella typhi. Chem. Eur. J. 2017, 23, 10670–10677. [Google Scholar] [CrossRef]
- Khan, M.I.; Soofi, S.B.; Ochiai, R.L.; Habib, M.A.; Sahito, S.M.; Nizami, S.Q.; Acosta, C.J.; Clemens, J.D.; Bhutta, Z.A.; DOMI Typhoid Karachi Vi Effectiveness Study Group. Effectiveness of vi capsular polysaccharide typhoid vaccine among children: A cluster randomized trial in karachi, pakistan. Vaccine 2012, 30, 5389–5395. [Google Scholar] [CrossRef] [PubMed]
- Szu, S.C. Development of vi conjugate—A new generation of typhoid vaccine. Expert Rev. Vaccines 2013, 12, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Rondini, S.; Pisoni, I.; Proietti, D.; Berti, F.; Costantino, P.; Rappuoli, R.; Szu, S.; Saul, A.; Martin, L.B. Vi-CRM197 as a new conjugate vaccine against salmonella typhi. Vaccine 2011, 29, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Gibani, M.M.; Moore, M.; Juel, H.B.; Jones, E.; Meiring, J.; Harris, V.; Gardner, J.; Nebykova, A.; Kerridge, S.A.; et al. Efficacy and immunogenicity of a vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of salmonella typhi: A randomised controlled, phase 2b trial. Lancet 2017, 390, 2472–2480. [Google Scholar] [CrossRef]
- Fusari, M.; Fallarini, S.; Lombardi, G.; Lay, L. Synthesis of di- and tri-saccharide fragments of salmonella typhi vi capsular polysaccharide and their zwitterionic analogues. Bioorg. Med. Chem. 2015, 23, 7439–7447. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.G.; Snelling, A.M. Pseudomonas aeruginosa: A formidable and ever-present adversary. J. Hosp. Infect. 2009, 73, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Pier, G.B. The challenges and promises of new therapies for cystic fibrosis. J. Exp. Med. 2012, 209, 1235–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Deng, Z.; Yan, A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.N.; Brook, I. Emerging resistant gram-negative aerobic bacilli in hospital-acquired infections. Chemotherapy 2010, 56, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Oleksiewicz, M.B.; Nagy, G.; Nagy, E. Anti-bacterial monoclonal antibodies: Back to the future? Arch. Biochem. Biophys. 2012, 526, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Thaden, J.T.; Keller, A.E.; Shire, N.J.; Camara, M.M.; Otterson, L.; Huband, M.; Guenther, C.M.; Zhao, W.; Warrener, P.; Stover, C.K.; et al. Pseudomonas aeruginosa bacteremic patients exhibit nonprotective antibody titers against therapeutic antibody targets pcrv and psl exopolysaccharide. J. Infect. Dis. 2016, 213, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and development of the pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009, 5, e1000354. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, S.; Wang, D.; Parsek, M.R.; Wozniak, D.J. The roles of biofilm matrix polysaccharide psl in mucoid pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 377–380. [Google Scholar] [CrossRef] [PubMed]
- DiGiandomenico, A.; Warrener, P.; Hamilton, M.; Guillard, S.; Ravn, P.; Minter, R.; Camara, M.M.; Venkatraman, V.; MacGill, R.S.; Lin, J.; et al. Identification of broadly protective human antibodies to pseudomonas aeruginosa exopolysaccharide psl by phenotypic screening. J. Exp. Med. 2012, 209, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Kocharova, N.A.; Knirel, Y.A.; Shashkov, A.S.; Kochetkov, N.K.; Pier, G.B. Structure of an extracellular cross-reactive polysaccharide from pseudomonas aeruginosa immunotype 4. J. Biol. Chem. 1988, 263, 11291–11295. [Google Scholar] [PubMed]
- Li, H.; Mo, K.F.; Wang, Q.; Stover, C.K.; DiGiandomenico, A.; Boons, G.J. Epitope mapping of monoclonal antibodies using synthetic oligosaccharides uncovers novel aspects of immune recognition of the psl exopolysaccharide of pseudomonas aeruginosa. Chem. Eur. J. 2013, 19, 17425–17431. [Google Scholar] [CrossRef] [PubMed]
- Crich, D.; Sun, S. Direct chemical synthesis of β-mannopyranosides and other glycosides via glycosyl triflates. Tetrahedron 1998, 54, 8321–8348. [Google Scholar] [CrossRef]
- Rosenstein, N.E.; Perkins, B.A.; Stephens, D.S.; Popovic, T.; Hughes, J.M. Meningococcal disease. N. Engl. J. Med. 2001, 344, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Harrison, O.B.; Claus, H.; Jiang, Y.; Bennett, J.S.; Bratcher, H.B.; Jolley, K.A.; Corton, C.; Care, R.; Poolman, J.T.; Zollinger, W.D.; et al. Description and nomenclature of neisseria meningitidis capsule locus. Emerg. Infect. Dis. 2013, 19, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Gotschlich, E.C.; Dunne, F.T.; Jonssen, E.K. Studies on the meningococcal polysaccharides: Ii. Composition and chemical properties of the group b and group c polysaccharide. J. Biol. Chem. 1971, 246, 4703–4712. [Google Scholar] [PubMed]
- Bhattacharjee, A.K.; Jennings, H.J.; Kenny, C.P.; Martin, A.; Smith, I.C. Structural determination of the polysaccharide antigens of neisseria meningitidis serogroups Y, W-135, and BO. Can. J. Biochem. 1976, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-Y.; Gotschlich, E.C.; Jonssen, E.K.; Wysocki, J.R. Studies on the meningococcal polysaccharides: I. Composition and chemical properties of the group a polysaccharide. J. Biol. Chem. 1971, 246, 2849–2858. [Google Scholar] [PubMed]
- Pon, R.A.; Lussier, M.; Yang, Q.-L.; Jennings, H.J. N-propionylated group b meningococcal polysaccharide mimics a unique bactericidal capsular epitope in group b neisseria meningitidis. J. Exp. Med. 1997, 185, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Farley, E.K.; Fusco, P.C.; Michon, F. Specificity of the immune response to a modified group b meningococcal polysaccharide conjugate vaccine. Clin. Vaccine Immunol. 2007, 14, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Tirani, M.; Meregaglia, M.; Melegaro, A. Health and economic outcomes of introducing the new menb vaccine (bexsero) into the italian routine infant immunisation programme. PLoS ONE 2015, 10, e0123383. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Findlow, J.; Borrow, R. Recombinant protein meningococcal serogroup b vaccine combined with outer membrane vesicles. Expert Opin Biol Ther 2011, 11, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.M.; Adu-Bobie, J.; Comanducci, M.; Aricò, B.; Savino, S.; Santini, L.; Brunelli, B.; Bambini, S.; Biolchi, A.; Capecchi, B.; et al. A universal vaccine for serogroup b meningococcus. Proc. Natl. Acad. Sci. USA 2006, 103, 10834–10839. [Google Scholar] [CrossRef] [PubMed]
- Coulson, G.B.; von Gottberg, A.; du Plessis, M.; Smith, A.M.; de Gouveia, L.; Klugman, K.P.; for the Group for Enteric, Respiratory; Meningeal Disease Surveillance in South Africa; Germs, S.A. Meningococcal disease in Siouth Afrca, 1999–2002. Emerg. Infect. Dis. 2007, 13, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Lee, J.-S.; Vázquez, J.A.; Enwere, G.; Taha, M.-K.; Kamiya, H.; Kim, H.M.; Jo, D.S. Meningococcal disease in the asia-pacific region: Findings and recommendations from the global meningococcal initiative. Vaccine 2016, 34, 5855–5862. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zaccaria, C.; Tontini, M.; Poletti, L.; Costantino, P.; Lay, L. Synthesis and preliminary biological evaluation of carba analogues from neisseria meningitidis a capsular polysaccharide. Org. Biomol. Chem. 2012, 10, 6673–6681. [Google Scholar] [CrossRef] [PubMed]
- Berkin, A.; Coxon, B.; Pozsgay, V. Towards a synthetic glycoconjugate vaccine against neisseria meningitidis a. Chem. Eur. J. 2002, 8, 4424–4433. [Google Scholar] [CrossRef]
- Slattegard, R.; Teodorovic, P.; Kinfe, H.H.; Ravenscroft, N.; Gammon, D.W.; Oscarson, S. Synthesis of structures corresponding to the capsular polysaccharide of neisseria meningitidis group a. Org. Biomol. Chem. 2005, 3, 3782–3787. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sanchez, M.I.; Zaccaria, C.; Buzzi, B.; Miglio, G.; Lombardi, G.; Polito, L.; Russo, G.; Lay, L. Synthesis and biological evaluation of phosphono analogues of capsular polysaccharide fragments from neisseria meningitidis a. Chem. Eur. J. 2007, 13, 6623–6635. [Google Scholar] [CrossRef] [PubMed]
- Teodorovic, P.; Slattegard, R.; Oscarson, S. Synthesis of stable c-phosphonate analogues of neisseria meningitidis group a capsular polysaccharide structures using modified mitsunobu reaction conditions. Org. Biomol. Chem. 2006, 4, 4485–4490. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Legnani, L.; Rencurosi, A.; Poletti, L.; Lay, L.; Russo, G. Modeling of synthetic phosphono and carba analogues of N-acetyl-α-D-mannosamine 1-phosphate, the repeating unit of the capsular polysaccharide from neisseria meningitidis serovar a. Org. Biomol. Chem. 2009, 7, 3734–3740. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Barbero, J.; Calloni, I.; Unione, L.; Jimenez-Oses, G.; Corzana, F.; Del Bino, L.; Corrado, A.; Pitirollo, O.; Colombo, C.; Lay, L.; et al. The conformation of the mannopyranosyl phosphate repeating unit of the capsular polysaccharide of Neisseria meningitides serogroup A and its carba-mimetic. Eur. J. Org. Chem. 2018, in press. [Google Scholar] [CrossRef]
- Nikolaev, A.V.; Botvinko, I.V.; Ross, A.J. Natural phosphoglycans containing glycosyl phosphate units: Structural diversity and chemical synthesis. Carbohydr. Res. 2007, 342, 297–344. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Tontini, M.; Brogioni, G.; Nilo, A.; Filippini, S.; Harfouche, C.; Polito, L.; Romano, M.R.; Costantino, P.; Berti, F.; et al. Immunoactivity of protein conjugates of carba analogues from neisseria meningitidis a capsular polysaccharide. ACS Chem. Biol. 2013, 8, 2561–2567. [Google Scholar] [CrossRef] [PubMed]
- Ramella, D.; Polito, L.; Mazzini, S.; Ronchi, S.; Scaglioni, L.; Marelli, M.; Lay, L. A strategy for multivalent presentation of carba analogues fromn. Meningitidisa capsular polysaccharide. Eur. J. Org. Chem. 2014, 2014, 5915–5924. [Google Scholar] [CrossRef]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Haacke, E.M. Principles, techniques, and applications of t2*-based mr imaging and its special applications. Radiographics 2009, 29, 1433–1449. [Google Scholar] [CrossRef] [PubMed]
- Bitar, R.; Leung, G.; Perng, R.; Tadros, S.; Moody, A.R.; Sarrazin, J.; McGregor, C.; Christakis, M.; Symons, S.; Nelson, A.; et al. MR pulse sequences: What every radiologist wants to know but is afraid to ask. RadioGraphics 2006, 26, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Jedlovszky-Hajdú, A.; Bombelli, F.B.; Monopoli, M.P.; Tombácz, E.; Dawson, K.A. Surface coatings shape the protein corona of spions with relevance to their application in vivo. Langmuir 2012, 28, 14983–14991. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sánchez, M.I.; Draghetti, V.; Panza, L.; Lay, L.; Russo, G. Synthesis of the phosphono analogue of the dimeric subunit of neisseria meningitidis type a capsular polysaccharide. Synlett 2005, 2005, 1147–1151. [Google Scholar] [CrossRef]
- Manea, F.; Bindoli, C.; Fallarini, S.; Lombardi, G.; Polito, L.; Lay, L.; Bonomi, R.; Mancin, F.; Scrimin, P. Multivalent, saccharide-functionalized gold nanoparticles as fully synthetic analogs of type a neisseria meningitidis antigens. Adv. Mater. 2008, 20, 4348–4352. [Google Scholar] [CrossRef]
- Fallarini, S.; Paoletti, T.; Battaglini, C.O.; Ronchi, P.; Lay, L.; Bonomi, R.; Jha, S.; Mancin, F.; Scrimin, P.; Lombardi, G. Factors affecting t cell responses induced by fully synthetic glyco-gold-nanoparticles. Nanoscale 2013, 5, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Fallarini, S.; Buzzi, B.; Giovarruscio, S.; Polito, L.; Brogioni, G.; Tontini, M.; Berti, F.; Adamo, R.; Lay, L.; Lombardi, G. A synthetic disaccharide analogue from neisseria meningitidis a capsular polysaccharide stimulates immune cell responses and induces immunoglobulin g production in mice when protein-conjugated. ACS Infect. Dis. 2015, 1, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Richmond, P.; Borrow, R.; Findlow, J.; Martin, S.; Thornton, C.; Cartwright, K.; Miller, E. Evaluation of de-o-acetylated meningococcal c polysaccharide-tetanus toxoid conjugate vaccine in infancy: Reactogenicity, immunogenicity, immunologic priming, and bactericidal activity against o-acetylated and de-o-acetylated serogroup c strains. Infect. Immun. 2001, 69, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.C.; Ren, C.T.; Lu, C.P.; Hsu, C.H.; Sun, T.H.; Han, J.L.; Pal, B.; Chao, T.A.; Lin, Y.F.; Wu, S.H.; et al. Efficient and stereoselective synthesis of alpha(2→9) oligosialic acids: From monomers to dodecamers. Angew. Chem. Int. Ed. Engl. 2011, 50, 9391–9395. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Zhou, Z.; Guo, Z. Synthesis and immunological study of alpha-2,9-oligosialic acid conjugates as anti-group c meningitis vaccines. Chem. Commun. (Camb.) 2015, 51, 9647–9650. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Zhou, Z.; Suryawanshi, S.; Mondal, M.A.; Guo, Z. Fully synthetic self-adjuvanting alpha-2,9-oligosialic acid based conjugate vaccines against group c meningitis. ACS Cent. Sci. 2016, 2, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Li, S.T.; Lin, T.L.; Cheng, Y.Y.; Sun, T.H.; Wang, J.T.; Cheng, T.J.R.; Mong, K.K.T.; Wong, C.H.; Wu, C.Y. Synthesis of neisseria meningitidis serogroup W135 capsular oligosaccharides for immunogenicity comparison and vaccine development. Angew. Chem. Int. Ed. 2013, 52, 9157–9161. [Google Scholar] [CrossRef] [PubMed]
- Delrieu, I.; Yaro, S.; Tamekloé, T.A.S.; Njanpop-Lafourcade, B.-M.; Tall, H.; Jaillard, P.; Ouedraogo, M.S.; Badziklou, K.; Sanou, O.; Drabo, A.; et al. Emergence of epidemic neisseria meningitidis serogroup x meningitis in togo and burkina faso. PLoS ONE 2011, 6, e19513. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yao, P.-P.; Zhang, L.-Y.; Li, Y.; Xu, F.; Mei, L.-L.; Zhu, S.-R.; Zhang, Y.-J.; Zhu, H.-P.; van der Veen, S. Capsule switching of neisseria meningitidis sequence type 7 serogroup a to serogroup x. J. Infect. 2017, 75, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Romano, M.R.; Tontini, M.; Cappelletti, E.; Gavini, M.; Proietti, D.; Rondini, S.; Swennen, E.; Santini, L.; Filippini, S.; et al. Development of a glycoconjugate vaccine to prevent meningitis in africa caused by meningococcal serogroup x. Proc. Natl. Acad. Sci. USA 2013, 110, 19077–19082. [Google Scholar] [CrossRef] [PubMed]
- Bundle, D.R.; Smith, I.C.P.; Jennings, H.J. Determination of the structure and conformation of bacterial polysaccharides by carbon 13 nuclear magnetic resonance: Studies on the group-specific antigens of neisseria meningitidis serogroups a and x. J. Biol. Chem. 1974, 249, 2275–2281. [Google Scholar] [PubMed]
- Xie, O.; Bolgiano, B.; Gao, F.; Lockyer, K.; Swann, C.; Jones, C.; Delrieu, I.; Njanpop-Lafourcade, B.-M.; Tamekloe, T.A.; Pollard, A.J.; et al. Characterization of size, structure and purity of serogroup x neisseria meningitidis polysaccharide, and development of an assay for quantification of human antibodies. Vaccine 2012, 30, 5812–5823. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Lay, L. Synthesis of neisseria meningitidis x capsular polysaccharide fragments. ARKIVOC Online J. Org. Chem. 2013, 166–184. [Google Scholar]
- Harale, K.R.; Dumare, N.B.; Singh, D.; Misra, A.K.; Chhikara, M.K. Synthesis of a tetrasaccharide and its glycoconjugate corresponding to the capsular polysaccharide of neisseria meningitidis serogroup x and its immunochemical studies. RSC Adv. 2015, 5, 41332–41340. [Google Scholar] [CrossRef]
- Oldrini, D.; Fiebig, T.; Romano, M.R.; Proietti, D.; Berger, M.; Tontini, M.; De Ricco, R.; Santini, L.; Morelli, L.; Lay, L.; et al. Combined chemical synthesis and tailored enzymatic elongation provide fully synthetic and conjugation-ready neisseria meningitidis serogroup x vaccine antigens. ACS Chem. Biol. 2018, 13, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Cancogni, D.; Tontini, M.; Nilo, A.; Filippini, S.; Costantino, P.; Romano, M.R.; Berti, F.; Adamo, R.; Lay, L. Synthesis and immunological evaluation of protein conjugates of neisseria meningitidis x capsular polysaccharide fragments. Beilstein J. Org. Chem. 2014, 10, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Tuberculosis. Available online: http://www.who.int/en/news-room/fact-sheets/detail/tuberculosis (accessed on 28 Febraury 2018).
- Lowary, T.L. Twenty years of mycobacterial glycans: Furanosides and beyond. Acc. Chem. Res. 2016, 49, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Veerapen, N.; Yuan, Y.; Sanders, D.A.; Pinto, B.M. Synthesis of novel ammonium and selenonium ions and their evaluation as inhibitors of udp-galactopyranose mutase. Carbohydr. Res. 2004, 339, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Huang, Z.; Liu, H.-W. Synthesis and analysis of substrate analogues for udp-galactopyranose mutase: Implication for an oxocarbenium ion intermediate in the catalytic mechanism. Org. Lett. 2007, 9, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Kuppala, R.; Borrelli, S.; Slowski, K.; Sanders, D.A.; Kartha, K.R.; Pinto, B.M. Synthesis and biological evaluation of nonionic substrate mimics of UDP-Galp as candidate inhibitors of udp galactopyranose mutase (UGM). Bioorg. Med. Chem. 2015, 25, 1995–1997. [Google Scholar] [CrossRef] [PubMed]
- Soltero-Higgin, M.; Carlson, E.E.; Phillips, J.H.; Kiessling, L.L. Identification of inhibitors for udp-galactopyranose mutase. J. Am. Chem. Soc. 2004, 126, 10532–10533. [Google Scholar] [CrossRef] [PubMed]
- Castagnolo, D.; De Logu, A.; Radi, M.; Bechi, B.; Manetti, F.; Magnani, M.; Supino, S.; Meleddu, R.; Chisu, L.; Botta, M. Synthesis, biological evaluation and sar study of novel pyrazole analogues as inhibitors of mycobacterium tuberculosis. Bioorg. Med. Chem. 2008, 16, 8587–8591. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Colombo, C.; Kuttiyatveetil, J.R.; Zalatar, N.; van Straaten, K.E.; Mohan, S.; Sanders, D.A.; Pinto, B.M. A second, druggable binding site in udp-galactopyranose mutase from mycobacterium tuberculosis? ChemBioChem 2016, 17, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Dykhuizen, E.C.; May, J.F.; Tongpenyai, A.; Kiessling, L.L. Inhibitors of udp-galactopyranose mutase thwart mycobacterial growth. J. Am. Chem. Soc. 2008, 130, 6706–6707. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, M.; Ronet, C.; Mempel, M.; Monsarrat, B.; Gachelin, G.; Puzo, G. Acylation state of the phosphatidylinositol mannosides from mycobacterium bovis bacillus calmette guérin and ability to induce granuloma and recruit natural killer t cells. J. Biol. Chem. 2001, 276, 34896–34904. [Google Scholar] [CrossRef] [PubMed]
- Torrelles, J.B.; Azad, A.K.; Schlesinger, L.S. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from mycobacterium tuberculosis by c-type lectin pattern recognition receptors. J. Immunol. 2006, 177, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Parlane, N.A.; Compton, B.J.; Hayman, C.M.; Painter, G.F.; Basaraba, R.J.; Heiser, A.; Buddle, B.M. Phosphatidylinositol di-mannoside and derivates modulate the immune response to and efficacy of a tuberculosis protein vaccine against mycobacterium bovis infection. Vaccine 2012, 30, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Stocker, B.L.; Seeberger, P.H. Total synthesis of phosphatidylinositol mannosides of mycobacterium tuberculosis. J. Am. Chem. Soc. 2006, 128, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Boonyarattanakalin, S.; Liu, X.; Michieletti, M.; Lepenies, B.; Seeberger, P.H. Chemical synthesis of all phosphatidylinositol mannoside (PIM) glycans from mycobacterium tuberculosis. J. Am. Chem. Soc. 2008, 130, 16791–16799. [Google Scholar] [CrossRef] [PubMed]
- Fraser-Reid, B.; Lu, J.; Jayaprakash, K.; Lopez, J.C. Synthesis of a 28-mer oligosaccharide core of mycobacterial lipoarabinomannan (LAM) requires only two n-pentenyl orthoester progenitors. Tetrahedron Asymmetry 2006, 17, 2449–2463. [Google Scholar] [CrossRef]
- Ali, A.; Wenk, M.R.; Lear, M.J. Total synthesis of a fully lipidated form of phosphatidyl-myo-inositol dimannoside (PIM-2) of mycobacterium tuberculosis. Tetrahedron Lett. 2009, 50, 5664–5666. [Google Scholar] [CrossRef]
- Patil, P.S.; Hung, S.-C. Synthesis of mycobacterial triacylated phosphatidylinositol dimannoside containing an acyl lipid chain at 3-o of inositol. Org. Lett. 2010, 12, 2618–2621. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; White, J.M.; Williams, S.J. Synthesis of glycoconjugate fragments of mycobacterial phosphatidylinositol mannosides and lipomannan. Beilstein J. Org. Chem. 2011, 7, 369. [Google Scholar] [CrossRef] [PubMed]
- Front, S.; Bourigault, M.-L.; Rose, S.; Noria, S.; Quesniaux, V.F.; Martin, O.R. Synthesis and biological investigation of pim mimics carrying biotin or a fluorescent label for cellular imaging. Bioconjug. Chem. 2012, 24, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.S.; Cheng, T.J.; Zulueta, M.M.; Yang, S.T.; Lico, L.S.; Hung, S.C. Total synthesis of tetraacylated phosphatidylinositol hexamannoside and evaluation of its immunomodulatory activity. Nat. Commun. 2015, 6, 7239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Xiong, D.-C.; Chen, S.-C.; Wang, Y.-S.; Ye, X.-S. Total synthesis of mycobacterial arabinogalactan containing 92 monosaccharide units. Nat. Commun. 2017, 8, 14851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahloul, K.; Lowary, T.L. Development of an orthogonal protection strategy for the synthesis of mycobacterial arabinomannan fragments. J. Org. Chem. 2015, 80, 11417–11434. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, S.; Wang, S.; Li, H.; Guo, Z.; Gu, G. Synthesis and immunological comparison of differently linked lipoarabinomannan oligosaccharide–monophosphoryl lipid a conjugates as antituberculosis vaccines. J. Org. Chem. 2017, 82, 12085–12096. [Google Scholar] [CrossRef] [PubMed]
- Parente-Rocha, J.A.; Bailão, A.M.; Amaral, A.C.; Taborda, C.P.; Paccez, J.D.; Borges, C.L. Antifungal resistance, metabolic routes as drug targets, and new antifungal agents: An overview about endemic dimorphic fungi. Mediat. Inflamm. 2017, 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A.R. An integrated model of the recognition of candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Cambi, A.; Gijzen, K.; de Vries, I.J.; Torensma, R.; Joosten, B.; Adema, G.J.; Netea, M.G.; Kullberg, B.J.; Romani, L.; Figdor, C.G. The c-type lectin dc-sign (CD209) is an antigen-uptake receptor for candida albicans on dendritic cells. Eur. J. Immunol. 2003, 33, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Gow, N.A.R.; Munro, C.A.; Bates, S.; Collins, C.; Ferwerda, G.; Hobson, R.P.; Bertram, G.; Hughes, H.B.; Jansen, T.; et al. Immune sensing of candida albicans requires cooperative recognition of mannans and glucans by lectin and toll-like receptors. J. Clin. Investig. 2006, 116, 1642–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.D.; Gordon, S. Immune recognition: A new receptor for β-glucans. Nature 2001, 413, 36. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.B.; Paulovičová, L.; Paulovičová, E.; Tsvetkov, Y.E.; Nifantiev, N.E. Recent advances in the synthesis of fungal antigenic oligosaccharides. Pure Appl. Chem. 2017, 89. [Google Scholar] [CrossRef]
- Richards, M.R.; Lowary, T.L. Chemistry and biology of galactofuranose-containing polysaccharides. ChemBioChem 2009, 10, 1920–1938. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.B.; Argunov, D.A.; Solovev, A.S.; Petruk, M.I.; Gerbst, A.G.; Dmitrenok, A.S.; Shashkov, A.S.; Latge, J.P.; Nifantiev, N.E. Synthesis of oligosaccharides related to galactomannans from aspergillus fumigatus and their nmr spectral data. Org. Biomol. Chem. 2018, 16, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Rex, J.H.; Lee, J.; Hamill, R.J.; Larsen, R.A.; Powderly, W.; Kauffman, C.A.; Hyslop, N.; Mangino, J.E.; Chapman, S.; et al. A prospective observational study of candidemia: Epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 2003, 37, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Bundle, D.R. The evolution of a glycoconjugate vaccine for candida albicans. In Carbohydrates as Drugs; Seeberger, P.H., Rademacher, C., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 187–234. [Google Scholar]
- Johnson, M.A.; Cartmell, J.; Weisser, N.E.; Woods, R.J.; Bundle, D.R. Molecular recognition of candida albicans (1→2)-β-mannan oligosaccharides by a protective monoclonal antibody reveals the immunodominance of internal saccharide residues. J. Biol. Chem. 2012, 287, 18078–18090. [Google Scholar] [CrossRef] [PubMed]
- Nycholat, C.M.; Bundle, D.R. Synthesis of monodeoxy and mono-O-methyl congeners of methyl β-D-mannopyranosyl-(1→2)-β-D-mannopyranoside for epitope mapping of anti-candida albicans antibodies. Carbohydr. Res. 2009, 344, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Nitz, M.; Ling, C.-C.; Otter, A.; Cutler, J.E.; Bundle, D.R. The unique solution structure and immunochemistry of thecandida albicans β-1,2-mannopyranan cell wall antigens. J. Biol. Chem. 2002, 277, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Nikrad, P.V.; Beierbeck, H.; Lemieux, R.U. Molecular recognition x. A novel procedure for the detection of the intermolecular hydrogen bonds present in a protein oligosaccharide complex. Can. J. Chem. 1992, 70, 241–253. [Google Scholar] [CrossRef]
- Bundle, D.R.; Nycholat, C.; Costello, C.; Rennie, R.; Lipinski, T. Design of a candida albicans disaccharide conjugate vaccine by reverse engineering a protective monoclonal antibody. ACS Chem. Biol. 2012, 7, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, T.; Wu, X.; Sadowska, J.; Kreiter, E.; Yasui, Y.; Cheriaparambil, S.; Rennie, R.; Bundle, D.R. A β-mannan trisaccharide conjugate vaccine aids clearance of candida albicans in immunocompromised rabbits. Vaccine 2012, 30, 6263–6269. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13526–13531. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kawai, T.; Adachi, Y.; Ohno, N.; Takahashi, T. [small beta](1,3) branched heptadeca- and linear hexadeca-saccharides possessing an aminoalkyl group as a strong ligand to dectin-1. Chem. Commun. 2010, 46, 8249–8251. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kawai, T.; Adachi, Y.; Hanashima, S.; Yamaguchi, Y.; Ohno, N.; Takahashi, T. Synthesis of beta(1,3) oligoglucans exhibiting a dectin-1 binding affinity and their biological evaluation. Bioorg. Med. Chem. 2012, 20, 3898–3914. [Google Scholar] [CrossRef] [PubMed]
- Bromuro, C.; Romano, M.; Chiani, P.; Berti, F.; Tontini, M.; Proietti, D.; Mori, E.; Torosantucci, A.; Costantino, P.; Rappuoli, R.; et al. Beta-glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine 2010, 28, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.; Tontini, M.; Brogioni, G.; Romano, M.R.; Costantini, G.; Danieli, E.; Proietti, D.; Berti, F.; Costantino, P. Synthesis of laminarin fragments and evaluation of a β-(1,3) glucan hexasaccaride-crm197 conjugate as vaccine candidate against candida albicans. J. Carbohydr. Chem. 2011, 30, 249–280. [Google Scholar] [CrossRef]
- Liao, G.; Zhou, Z.; Burgula, S.; Liao, J.; Yuan, C.; Wu, Q.; Guo, Z. Synthesis and immunological studies of linear oligosaccharides of beta-glucan as antigens for antifungal vaccine development. Bioconjug. Chem. 2015, 26, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Zhou, Z.; Liao, J.; Zu, L.; Wu, Q.; Guo, Z. 6-O-branched oligo-β-glucan-based antifungal glycoconjugate vaccines. ACS Infect. Dis. 2016, 2, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Komarova, B.S.; Orekhova, M.V.; Tsvetkov, Y.E.; Beau, R.; Aimanianda, V.; Latge, J.P.; Nifantiev, N.E. Synthesis of a pentasaccharide and neoglycoconjugates related to fungal alpha-(1→3)-glucan and their use in the generation of antibodies to trace aspergillus fumigatus cell wall. Chem. Eur. J. 2015, 21, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P.; Kobayashi, H.; Debeaupuis, J.P.; Diaquin, M.; Sarfati, J.; Wieruszeski, J.M.; Parra, E.; Bouchara, J.P.; Fournet, B. Chemical and immunological characterization of the extracellular galactomannan of aspergillus fumigatus. Infect. Immun. 1994, 62, 5424–5433. [Google Scholar] [PubMed]
- Kudoh, A.; Okawa, Y.; Shibata, N. Significant structural change in both O- and N-linked carbohydrate moieties of the antigenic galactomannan from aspergillus fumigatus grown under different culture conditions. Glycobiology 2015, 25, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Argunov, D.A.; Krylov, V.B.; Nifantiev, N.E. Convergent synthesis of isomeric heterosaccharides related to the fragments of galactomannan from aspergillus fumigatus. Org. Biomol. Chem. 2015, 13, 3255–3267. [Google Scholar] [CrossRef] [PubMed]
- Argunov, D.A.; Krylov, V.B.; Nifantiev, N.E. The use of pyranoside-into-furanoside rearrangement and controlled O(5)→O(6) benzoyl migration as the basis of a synthetic strategy to assemble (1→5)- and (1→6)-linked galactofuranosyl chains. Org. Lett. 2016, 18, 5504–5507. [Google Scholar] [CrossRef] [PubMed]
- Matveev, A.L.; Krylov, V.B.; Emelyanova, L.A.; Solovev, A.S.; Khlusevich, Y.A.; Baykov, I.K.; Fontaine, T.; Latgé, J.-P.; Tikunova, N.V.; Nifantiev, N.E. Novel mouse monoclonal antibodies specifically recognize aspergillus fumigatus galactomannan. PLoS ONE 2018, 13, e0193938. [Google Scholar] [CrossRef] [PubMed]
- Kabat, E.A. The upper limit for the size of the human antidextran combining site. J. Immunol. 1960, 84, 82–85. [Google Scholar] [PubMed]
- Nilo, A.; Morelli, L.; Passalacqua, I.; Brogioni, B.; Allan, M.; Carboni, F.; Pezzicoli, A.; Zerbini, F.; Maione, D.; Fabbrini, M.; et al. Anti-group b streptococcus glycan-conjugate vaccines using pilus protein gbs80 as carrier and antigen: Comparing lysine and tyrosine-directed conjugation. ACS Chem. Biol. 2015, 10, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, G.; Hu, Q.-Y.; Usera, A.; Robinson, Z.; Allan, M.; Singh, A.; Imase, H.; Cobb, J.; Zhai, H.; Quinn, D.; et al. Sugar-protein connectivity impacts on the immunogenicity of site-selective salmonella o-antigen glycoconjugate vaccines. Angew. Chem. Int. Ed. 2015, 54, 13198–13203. [Google Scholar] [CrossRef]
- Fernandez-Tejada, A.; Canada, F.J.; Jimenez-Barbero, J. Recent developments in synthetic carbohydrate-based diagnostics, vaccines, and therapeutics. Chem. Eur. J. 2015, 21, 10616–10628. [Google Scholar] [CrossRef] [PubMed]

































© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, C.; Pitirollo, O.; Lay, L. Recent Advances in the Synthesis of Glycoconjugates for Vaccine Development. Molecules 2018, 23, 1712. https://doi.org/10.3390/molecules23071712
Colombo C, Pitirollo O, Lay L. Recent Advances in the Synthesis of Glycoconjugates for Vaccine Development. Molecules. 2018; 23(7):1712. https://doi.org/10.3390/molecules23071712
Chicago/Turabian StyleColombo, Cinzia, Olimpia Pitirollo, and Luigi Lay. 2018. "Recent Advances in the Synthesis of Glycoconjugates for Vaccine Development" Molecules 23, no. 7: 1712. https://doi.org/10.3390/molecules23071712
APA StyleColombo, C., Pitirollo, O., & Lay, L. (2018). Recent Advances in the Synthesis of Glycoconjugates for Vaccine Development. Molecules, 23(7), 1712. https://doi.org/10.3390/molecules23071712