A Malonyl-Based Scaffold for Conjugatable Multivalent Carbohydrate-BODIPY Presentations
Abstract
:1. Introduction
2. Results and Discussion
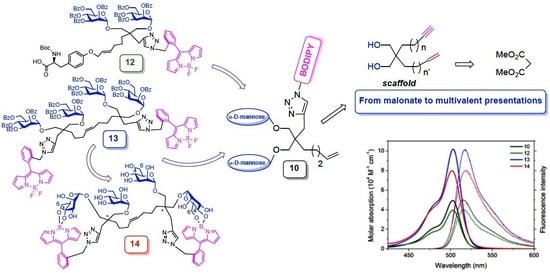
2.1. Synthesis, Glycosylation, and CuAAC Reaction of The Scaffold
2.2. Cross-Metathesis Reactions
2.3. De-O-Benzoylation of Dimer 13
2.4. Photophysical Studies
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedure for Alkylation
3.1.2. General Procedure for the Cross-Metathesis (CM) Reaction
3.2. Spectroscopy
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Varki, A.; Cummings, R.; Esko, J.; Freeze, H.; Hart, G.; Marth, J. Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1999. [Google Scholar]
- Jayaraman, N. Multivalent ligand presentation as a central concept to study intricate carbohydrate-protein interactions. Chem. Soc. Rev. 2009, 38, 3463–3483. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.D.; Raines, R.T. Bright Ideas for Chemical Biology. ACS Chem. Biol. 2008, 3, 142–155. [Google Scholar] [CrossRef] [Green Version]
- Davis, L.D.; Raines, R.T. Bright Building Blocks for Chemical Biology. ACS Chem. Biol. 2014, 9, 855–866. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Soh, N. Selective Chemical Labeling of Proteins with Small Fluorescent Molecules Based on Metal-Chelation Methodology. Sensors 2008, 8, 1004–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.-L.; Xu, Z.; Yang, Y.; Xu, L. Small-molecule fluorescent probes for specific detection and imaging of chemical species inside lysosomes. Chem. Commun. 2019. accepted manuscript. [Google Scholar] [CrossRef]
- Chilka, P.; Desai, N.; Datta, B. Small molecule fluorescent probes for G-quadruplex visualization as potential cancer theranostic agents. Molecules 2019, 24, 752. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Nagano, T. Fluorescent probes for sensing and imaging. Nat. Methods 2011, 8, 642–645. [Google Scholar] [CrossRef]
- Fernandez-Suarez, M.; Ting, A.Y. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. 2008, 9, 929–943. [Google Scholar] [CrossRef]
- Mechref, Y.; Novotny, M.V. Structural investigations of glycoconjugates at high sensititvity. Chem. Rev. 2002, 102, 321–369. [Google Scholar] [CrossRef]
- Xia, B.; Kawar, Z.S.; Ju, T.; Alvarez, R.A.; Sachdev, G.P.; Cummings, R.D. Versatile fluorescent derivatization of glycans for glycomic analysis. Nat. Methods 2005, 2, 845–850. [Google Scholar] [CrossRef]
- Lohse, A.; Martins, R.; Jørgensen, M.R.; Hindsgaul, O. Solid-Phase Oligosaccharide Tagging (SPOT): Validation on Glycolipid-Derived Structures. Angew. Chem. Int. Ed. 2006, 45, 4167–4172. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.M.H.; Chee, S.H.; Knight, D.A.; Acha-Orbea, H.; Hermans, I.F.; Timmer, M.S.M.; Stocker, B.L. An improved synthesis of dansylated α-galactosylceramide and its use as a fluorescent probe for the monitoring of glycolipid uptake by cells. Carbohydr. Res. 2011, 346, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Han, J.H.; Kwon, P.-S.; Bhuniya, S.; Kim, J.Y.; Sessler, J.L.; Kang, C.; Kim, J.S. Hepatocyte-Targeting Single Galactose-Appended Naphthalimide: A Tool for Intracellular Thiol Imaging in Vivo. J. Am. Chem. Soc. 2012, 134, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.-T.; Zhang, Y.; Lv, Y.; Wu, J.; Zang, Y.; Tan, C.; Li, J.; Chen, G.-R.; He, X.P. Interlocked supramolecular glycoconjugated polymers for receptor-targeting theranostics. Chem. Commun. 2016, 52, 3821–3824. [Google Scholar] [CrossRef]
- Wahiba, M.; Feng, X.Q.; Zang, Y.; James, T.D.; Li, J.; Chen, G.-R.; He, X.P. A supramolecular pyrenyl glycoside-coated 2D MoS2 composite electrode for selective cell capture. Chem. Commun. 2016, 52, 11689–11692. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, Y.; Li, Q.-Y.; Hao, L.-N.; Ma, Z.; Wang, X.-J.; Yin, J. Targeted delivery of a Mannose-conjugated BODIPY photosensitizer by nanomicelles for photodynamic breast cancer therapy. Chem. Eur. J. 2017, 23, 14307–14315. [Google Scholar] [CrossRef]
- Ribeiro-Viana, R.; Garcia-Vallejo, J.J.; Collado, D.; Perez-Inestrosa, E.; Bloem, K.; van Kooyk, Y.; Rojo, J. BODIPY-Labeled DC-SIGN-Targeting Glycodendrons Efficiently Internalize and Route to Lysosomes in Human Dendritic Cells. Biomacromolecules 2012, 13, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.; Hoogendoorn, S.; van der Marel, G.A.; Overkleeft, H.S.; Codee JD, C. Targeted Delivery of Fluorescent High-Mannose-Type Oligosaccharide Cathepsin Inhibitor Conjugates. ChemPlusChem 2015, 80, 928–937. [Google Scholar] [CrossRef]
- Shi, D.-T.; Zhou, D.; Zang, Y.; Li, J.; Chen, G.-R.; James, T.D.; He, X.P.; Tian, H. Selective fluorogenic imaging of hepatocellular H2S by a galactosyl azidonaphthalimide probe. Chem. Commun. 2015, 51, 3653–3655. [Google Scholar] [CrossRef] [PubMed]
- Calatrava-Perez, E.; Bright, S.A.; Achermann, S.; Moylan, C.; Senge, M.O.; Veale, E.B.; Williams, D.C.; Gunlaugsson, T.; Scanlan, E.M. Glycosidase activated release of fluorescent 1,8-naphthalimide probes for tumor cell imaging from glycosylated “pro-probes”. Chem. Commun. 2016, 52, 13086–13089. [Google Scholar] [CrossRef]
- Dong, L.; Zang, Y.; Zhou, D.; He, X.-P.; Chen, G.-R.; James, T.D.; Li, J. Glycosylation enhances the aqueous sensitivity and lowers the cytotoxicity of a naphthalimide zinc ion fluorescence probe. Chem. Commun. 2015, 51, 11852–11855. [Google Scholar] [CrossRef]
- Liu, F.; Tang, P.; Ding, R.; Liao, L.; Wang, L.; Wang, M.; Wang, J. A glycosylation strategy to develop a low toxic naphthalimide fluorescent probe for the detection of Fe3+ in aqueous medium. Dalton Trans. 2017, 46, 7515–7522. [Google Scholar] [CrossRef]
- Lee, Y.C.; RT Lee, R.T. Carbohydrate-protein interactions: Basis of glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Fasting, C.; Schalley, C.A.; Weber, M.; Seitz, O.; Hecht, S.; Koksch, B.; Dernedde, J.; Graf, C.; Knapp, E.-W.; Haag, R. Multivalency as a Chemical Organization and Action Principle. Angew. Chem. Int. Ed. 2012, 51, 10472–10498. [Google Scholar] [CrossRef] [Green Version]
- Quadir, M.; Fehse, S.; Multhaup, G.; Haag, R. Hyperbranched Polyglycerol Derivatives as Prospective Copper Nanotransporter Candidates. Molecules 2018, 23, 1281. [Google Scholar] [CrossRef]
- Gestwicki, J.E.; Strong, L.E.; Cairo, C.W.; Boehm, F.J.; Kiessling, L.L. Cell Aggregation by Scaffolded Receptor Clusters. Chem. Biol. 2002, 9, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, A.; Jimenez-Barbero, J.; Casnati, A.; De Castro, C.; Darbre, T.; Fieschi, F.; Finne, J.; Funken, H.; Jaeger, K.-E.; Lahmann, M.; et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013, 42, 4709–4727. [Google Scholar] [CrossRef] [PubMed]
- Pagé, D.; Zanini, D.; Roy, R. Macromolecular Recognition: Effect of Multivalency in the Inhibition of Binding of Yeast Mannan to Concanavalin A and Pea Lectins by Mannosylated Dendrimers. Bioorg. Med. Chem. 1996, 4, 1949–1961. [Google Scholar] [CrossRef]
- Sanchez-Navarro, M.; Munoz, A.; Illescas, B.M.; Rojo, J.; Martin, N. [60]Fullerene as Multivalent Scaffold: Efficient Molecular Recognition of Globular Glycofullerenes by Concanavalin A. Chem. Eur. J. 2011, 17, 766–769. [Google Scholar] [CrossRef]
- Illescas, B.M.; Rojo, J.; Delgado, R.; Martin, N. Multivalent Glycosylated Nanostructures to Inhibit Ebola Virus Infection. J. Am. Chem. Soc. 2017, 139, 6018–6025. [Google Scholar] [CrossRef] [PubMed]
- Diwan, D.; Shinkai, K.; Tetsuka, T.; Cao, B.; Arai, H.; Koyama, T.; Hatano, K.; Matsuoka, K. Synthetic Assembly of Mannose Moieties Using Polymer Chemistry and the Biological Evaluation of Its Interaction towards Concanavalin A. Molecules 2017, 22, 157. [Google Scholar] [CrossRef]
- Wojcik, F.; O’Brien, A.G.; Götze, S.; Seeberger, P.H.; Hartmann, L. Synthesis of Carbohydrate-Functionalised Sequence-Defined Oligo(amidoamine)s by Photochemical Thiol–Ene Coupling in a Continuous Flow Reactor. Chem. Eur. J. 2013, 19, 3090–3098. [Google Scholar] [CrossRef] [PubMed]
- Bücher, K.S.; Konietzny, P.B.; Snyder, N.L.; Hartmann, L. Heteromultivalent Glycooligomers as Mimetics of Blood Group Antigens. Chem. Eur. J. 2019, 25, 3301–3309. [Google Scholar] [CrossRef]
- Papp, I.; Dernedde, J.; Enders, S.; Riese, S.B.; Shiao, T.C.; Roy, R.; Haag, R. Multivalent Presentation of Mannose on Hyperbranched Polyglycerol and their Interaction with Concanavalin a Lectin. ChemBioChem 2011, 12, 1075–1083. [Google Scholar] [CrossRef]
- Chabre, Y.M.; Roy, R. Design and creativity in synthesis of multivalent glycoconjugates. Adv. Carbohydr. Chem. Biochem. 2010, 63, 165–393. [Google Scholar] [PubMed]
- Bojarova, P.; Kren, V. Sugared biomaterial binding lectins: Achievements and perspectives. Biomater. Sci. 2016, 4, 1142–1160. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.R.; Urias-Benavides, A.; Alvarado-Martinez, E.; Lopez, J.C.; Gomez, A.M.; del Rio, M.; Garcia, I.; Costela, A.; Bañuelos, J.; Arbeloa, T.; et al. Convenient Access to Carbohydrate–BODIPY Hybrids by Two Complementary Methods Involving One-Pot Assembly of “Clickable” BODIPY Dyes. Eur. J. Org. Chem. 2014, 5659–5663. [Google Scholar] [CrossRef]
- Del Rio, M.; Lobo, F.; López, J.C.; Oliden, A.; Bañuelos, J.; López-Arbeloa, I.; García-Moreno, I.; Gómez, A.M. One-pot synthesis of rotationally restricted, conjugatable, BODIPY derivatives from phtalides. J. Org. Chem. 2017, 82, 1240–1247. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Li, L.; Han, J.; Nguyen, B.; Burgess, K. Syntheses and spectral properties of functionalized, water-soluble BODIPY derivatives. J. Org. Chem. 2008, 73, 1963–1970. [Google Scholar] [CrossRef]
- Poirel, A.; Retailleau, P.; De Nicola, A.; Ziessel, R. Synthesis of Water-Soluble Red-Emitting Thienyl–BODIPYs and Bovine Serum Albumin Labeling. Chem. Eur. J. 2014, 20, 1252–1257. [Google Scholar] [CrossRef]
- Niu, S.L.; Ulrich, G.; Ziessel, R.; Kiss, A.; Renard, P.-Y.; Romieu, A. Water-soluble BODIPY derivatives. Org. Lett. 2009, 11, 2049–2052. [Google Scholar] [CrossRef]
- He, X.-H.; Zang, Y.; James, T.D.; Li, J.; Chen, G.-R.; Xie, J. Fluorescent glycoprobes: A sweet addition for improved sensing. Chem. Commun. 2017, 52, 82–90. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-Based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Lee, Y.C. Synthesis of some cluster glycosides suitable for attachment to proteins or solid matrices. Carbohydr. Res. 1978, 67, 509–514. [Google Scholar]
- Chabre, Y.M.; Roy, R. Multivalent glycoconjugate syntheses and applications using aromatic scaffolds. Chem. Soc. Rev. 2013, 42, 4657–4708. [Google Scholar] [CrossRef]
- Ivanova, I.M.; Negopodiev, S.A.; Saalbach, G.; O’Neill, E.C.; Urbaniak, M.D.; Ferguson MA, J.; Gurcha, S.S.; Besra, G.S.; Field, R.A. Fluorescent mannosides serve as acceptor substrates for glycosyltransferase and sugar-1-phosphate transferase activities in Euglena gracilis membranes. Carbohydr. Res. 2017, 438, 26–38. [Google Scholar] [CrossRef]
- Ribeiro-Viana, R.; Sanchez-Navarro, M.; Luckowiak, J.; Koeppe, J.R.; Delgado, R.; Rojo, J.; Davis, B.G. Virus-like glycodendrinanoparticles displaying quasi-equivalent nested polyvalency upon glycoprotein platforms potently block viral infection. Nat. Commun. 2012, 3, 1303–1311. [Google Scholar] [CrossRef]
- Guo, Y.; Feinberg, H.; Conroy, E.; Mitchell, D.A.; Alvarez, R.; Blixt, O.; Taylor, M.E.; Weis, W.I.; Drickamer, K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 2004, 11, 591–598. [Google Scholar] [CrossRef]
- Ehrmann, S.; Chu, C.-W.; Kumari, S.; Silberreis, K.; Bottcher, C.; Dernedde, J.; Ravoo, B.J.; Haag, R. A toolbox approach for multivalent presentation of ligand-receptor recognition on a supramolecular scaffold. J. Mater. Chem. B 2018, 6, 4216–4222. [Google Scholar] [CrossRef]
- Meldal, M.; Tornoe, C.W. Cu-Catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Grubbs, R.H. Handbook of Metathesis; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Aljarilla, A.; López, J.C.; Plumet, J. Metathesis Reactions of Carbohydrates: Recent Highlights in Cross-Metathesis. Eur. J. Org. Chem. 2010, 6123–6143. [Google Scholar] [CrossRef]
- Carruthers, W.; Coldham, I. Modern Methods of Organic Synthesis, 4th ed.; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Uriel, C.; Ventura, J.; Gómez, A.M.; López, J.C.; Fraser-Reid, B. Methyl 1,2-Orthoesters as Useful Glycosyl Donors in Glycosylation Reactions: A Comparison with n-Pent-4-enyl 1,2-Orthoesters. Eur. J. Org. Chem. 2012, 3122–3131. [Google Scholar] [CrossRef]
- Uriel, C.; Rijo, P.; Fernandes, A.S.; Gómez, A.M.; Fraser-Reid, B.; López, J.C. Methyl 1,2-Orthoesters in Acid-Washed Molecular Sieves Mediated Glycosylations. ChemistrySelect 2016, 1, 6011–6015. [Google Scholar] [CrossRef]
- Liu, B.; Novikova, N.; Simpson, M.C.; Timmer MS, M.; Stocker, B.L.; Sohnel, T.; Ware, D.C.; Brothers, P.J. Lighting up sugars: Fluorescent BODIPY–glucofuranose and –septanose conjugates linked by direct B–O–C bonds. Org. Biomol. Chem. 2016, 14, 5205–5209. [Google Scholar] [CrossRef]
- Esnal, I.; Valois-Escamilla, I.; Gómez-Durán, C.F.A.; Urías-Benavides, A.; Betancourt-Mendiola, M.L.; López-Arbeloa, I.; Bañuelos, J.; García-Moreno, I.; Costela, A.; Peña-Cabrera, E. Blue-to-orange color-tunable laser emission from tailored boron-dipyrromethene dyes. ChemPhysChem 2013, 14, 4134–4142. [Google Scholar] [CrossRef]
- Kee, H.L.; Kirmaier, C.; Yu, L.; Thamyongkit, P.; Youngblood, W.J.; Calder, M.E.; Ramos, L.; Noll, B.C.; Bocian, D.F.; Scheidt, W.R.; et al. Structural control of the photodynamics of boron-dipyrrin complexes. J. Phys. Chem. B 2005, 109, 20433–20443. [Google Scholar] [CrossRef]
- Durán-Sampedro, G.; Agarrabeitia, A.A.; Cerdán, L.; Pérez-Ojeda, M.E.; Costela, A.; García-Moreno, I.; Esnal, I.; Bañuelos, J.; López-Arbeloa, I.; Ortiz, M.J. Carboxylates versus fluorines: Boosting the emission properties of commercial BODIPYs in liquid and solid media. Adv. Funct. Mater. 2013, 23, 4195–4205. [Google Scholar] [CrossRef]
- Kammerer, C.; Prestat, G.; Gaillard, T.; Madec, D.; Poli, G. A Successful Cohabitation of Pd and Ru. Org. Lett. 2008, 10, 405–408. [Google Scholar] [CrossRef] [PubMed]





| Dye | Solvent | λab (nm) | εmax (104 M−1 cm−1) | λfl (nm) | ϕ | τ (ns) |
|---|---|---|---|---|---|---|
| 10 | Ethyl acetate | 502.5 | 5.0 | 515.5 | 0.90 | 7.02 |
| Acetone | 502.5 | 5.1 | 515.0 | 0.80 | 7.05 | |
| Acetonitrile | 502.0 | 5.0 | 515.0 | 0.82 | 7.53 | |
| 12 | Ethyl acetate | 503.0 | 4.0 | 516.0 | 0.78 | 6.14 |
| Acetone | 502.5 | 4.2 | 516.5 | 0.55 | 5.04 | |
| Acetonitrile | 502.5 | 3.5 | 515.5 | 0.60 | 5.20 | |
| 13 | Ethyl acetate | 503.0 | 10.2 | 518.0 | 0.70 | 5.69 |
| Acetone | 503.0 | 9.3 | 517.5 | 0.50 | 5.04 | |
| Acetonitrile | 502.5 | 9.5 | 516.0 | 0.55 | 5.14 | |
| 14 | Methanol | 501.5 | 8.5 | 518.0 | 0.40 | 4.38 |
| Water | 501.5 | 8.0 | 518.0 | 0.30 | 3.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uriel, C.; Sola-Llano, R.; Bañuelos, J.; Gomez, A.M.; Lopez, J.C. A Malonyl-Based Scaffold for Conjugatable Multivalent Carbohydrate-BODIPY Presentations. Molecules 2019, 24, 2050. https://doi.org/10.3390/molecules24112050
Uriel C, Sola-Llano R, Bañuelos J, Gomez AM, Lopez JC. A Malonyl-Based Scaffold for Conjugatable Multivalent Carbohydrate-BODIPY Presentations. Molecules. 2019; 24(11):2050. https://doi.org/10.3390/molecules24112050
Chicago/Turabian StyleUriel, Clara, Rebeca Sola-Llano, Jorge Bañuelos, Ana M. Gomez, and J. Cristobal Lopez. 2019. "A Malonyl-Based Scaffold for Conjugatable Multivalent Carbohydrate-BODIPY Presentations" Molecules 24, no. 11: 2050. https://doi.org/10.3390/molecules24112050
APA StyleUriel, C., Sola-Llano, R., Bañuelos, J., Gomez, A. M., & Lopez, J. C. (2019). A Malonyl-Based Scaffold for Conjugatable Multivalent Carbohydrate-BODIPY Presentations. Molecules, 24(11), 2050. https://doi.org/10.3390/molecules24112050