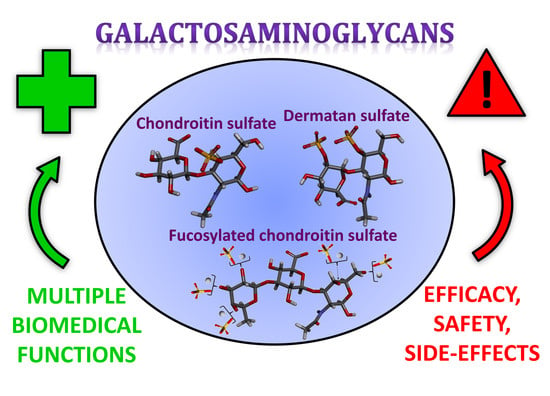
Galactosaminoglycans: Medical Applications and Drawbacks
Abstract
:1. Introduction
2. CS
2.1. Overview
2.2. Biomedical Properties
2.2.1. Inflammation
2.2.2. Cancer and Metastasis
2.2.3. Neural Growth Stimulation and Inhibition
2.2.4. Antiviral and Antibacterial Activity
3. DS
3.1. Overview
3.2. Biomedical Properties
3.2.1. Coagulation and Thrombosis
3.2.2. Wound Healing
3.2.3. Inflammation
3.2.4. Cancer and Metastasis
3.2.5. Antiviral Activity
4. FucCS
4.1. Overview
4.2. Biomedical Properties
4.2.1. Coagulation and Thrombosis
4.2.2. Hemodialysis
4.2.3. Atherosclerosis
4.2.4. Cellular Growth
4.2.5. Angiogenesis
4.2.6. Fibrosis
4.2.7. Cancer and Inflammation
4.2.8. Microbial Infections
Viral Infections
Malaria-Related Protozoan Infection
4.2.9. Hyperglycemia Diabetes-Related Processes
Diabetic Nephropathy
Diabetes-Related Apoptosis of Islets of Langerhans
4.2.10. Tissue Damage
5. Drawbacks
5.1. The Dilemma Regarding Effectiveness and Safety of CS as a Dietary Supplement for Treatments of Osteoarthritis
5.2. The Presence of Unexpected Components in CS-Based Pharmaceutical Formulations for Oral Administration
5.3. Potential Antigenic and Autoimmune Side-Effects of CS
5.4. Highly Sulfated CS can Activate the Kinin-Kallikrein System
5.5. Potential Problems for the Medical Use of DS and other GalAGs
5.6. Potential Harmful Effects of FucCS
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pomin, V.H.; Mulloy, B. Glycosaminoglycans and Proteoglycans. Pharmaceuticals 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Silbert, J.E.; Sugumaran, G. Biosynthesis of Chondroitin/Dermatan Sulfate. IUBMB Life 2002, 54, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Mikami, T.; Uyama, T.; Mizuguchi, S.; Nomura, K.; Kitagawa, H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003, 13, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Tanaka, Y.; Yamada, S.; Seno, N.; Haslam, S.M.; Morris, H.R.; Dell, A.; Sugahara, K. A Novel Pentasaccharide Sequence GlcA(3-sulfate)(β1-3)GalNAc(4-sulfate)(β1-4)(FucR1-3)GlcA(β1-3)GalNAc(4-sulfate) in the Oligosaccharides Isolated from King Crab Cartilage Chondroitin Sulfate K and Its Differential Susceptibility to Chondroitinases and Hyaluronidase. Biochemistry 1997, 2960, 3998–4008. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mourão, P.A. Occurrence of a unique fucose-branched chondroitin sulfate in the body wall of a sea cucumber. J. Biol. Chem. 1988, 263, 18176–18183. [Google Scholar]
- Pomin, V.H. Holothurian Fucosylated Chondroitin Sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomin, V.H.; Sharp, J.S.; Li, X.; Wang, L.; Prestegard, J.H. Characterization of Glycosaminoglycans by 15 N NMR Spectroscopy and in Vivo Isotopic Labeling. Anal Chem. 2010, 82, 4078–4088. [Google Scholar] [CrossRef]
- Sirko, S.; Von Holst, A.; Wizenmann, A.; Götz, M.; Faissner, A. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development 2007, 2738, 2727–2738. [Google Scholar] [CrossRef]
- Gu, W.; Fu, S.; Wang, Y.; Li, Y.; Lü, H.; Xu, M.; Lu, P. Chondroitin sulfate proteoglycans regulate the growth, differentiation and migration of multipotent neural precursor cells through the integrin signaling pathway. BMC Neurosci. 2009, 15. [Google Scholar] [CrossRef]
- Tanaka, M.; Maeda, N.; Noda, M.; Marunouchi, T. A Chondroitin Sulfate Proteoglycan PTP ζ/RPTPβ Regulates the Morphogenesis of Purkinje Cell Dendrites in the Developing Cerebellum. J. Neurosci. 2003, 23, 2804–2814. [Google Scholar] [CrossRef]
- Shannon, J.M.; Mccormick-Shannon, K.; Burhans, M.S.; Shangguan, X.; Srivastava, K.; Hyatt, B.A. Chondroitin sulfate proteoglycans are required for lung growth and morphogenesis in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L1323–L1336. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.C.; Solursh, M. Primary mesenchyme cell migration requires a chondroitin sulfate/dermatan sulfate proteoglycan. Dev. Biol. 1991, 143, 389–397. [Google Scholar] [CrossRef]
- Villena, J.; Brandan, E. Dermatan Sulfate Exerts an Enhanced Growth Factor Response on Skeletal Muscle Satellite Cell Proliferation and Migration. J. Cell. Physiol. 2004, 178, 169–178. [Google Scholar] [CrossRef]
- Higgins, M.K. The Structure of a Chondroitin Sulfate-binding Domain Important in Placental Malaria. J. Biol. Chem. 2008, 283, 21842–21846. [Google Scholar] [CrossRef] [Green Version]
- Tollefsen, D.M. Activation of heparin cofactor II by heparin and dermatan sulfate. Nouv. Rev. Fr. Hematol. 1984, 26, 233–237. [Google Scholar] [CrossRef]
- Tollefsen, D.M. Vascular Dermatan Sulfate and Heparin Cofactor II. In Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2010; pp. 351–372. [Google Scholar]
- Raghuraman, A.; Mosier, P.D.; Desai, U.R. Understanding Dermatan Sulfate-Heparin Cofactor II Interaction through Virtual Library Screening. ACS Med. Chem. Lett. 2010, 1, 281–285. [Google Scholar] [CrossRef]
- Vicente, C.P.; Zancan, P.; Peixoto, L.L.; Alves-Sá, R.; Araújo, F.S.; Mourão, P.A.S.; Pavão, M.S.G. Unbalanced effects of dermatan sulfates with different sulfation patterns on coagulation, thrombosis and bleeding. Thromb. Haemost. 2001, 86, 1215–1220. [Google Scholar] [CrossRef]
- Mourão, P.A.S.; Pavão, M.S.G.; Mulloy, B.; Tollefsen, D.M.; Mowinckel, M.; Abildgaard, U. Structure and Anticoagulant Activity of a Fucosylated Chondroitin Sulfate from Echinoderm. Sulfated fucose branches on the polysaccharide account for its high anticoagulant action. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef]
- Prandoni, P.; Meduri, F.; Cuppini, S.; Toniato, A.; Zangrandi, F. Dermatan sulphate: A safe approach to prevention of postoperative deep vein thrombosis. Br. J. Surg. 1992, 79, 505–509. [Google Scholar] [CrossRef]
- Mourão, P.A.S.; Guimarães, M.A.M.; Mulloy, B.; Thomas, S.; Gray, E. Antithrombotic activity of a fucosylated chondroitin sulphate from echinoderm: Sulphated fucose branches on the polysaccharide account for its antithrombotic action. Br. J. Haematol. 1998, 101, 647–652. [Google Scholar] [CrossRef]
- Iovu, M.M.D.; Dumais, G.B.S.; Souich, P.M.D. Anti-inflammatory activity of chondroitin sulfate. Osteoarthr. Cartil. 2008, 16, 14–18. [Google Scholar] [CrossRef]
- Volpi, N. Anti-inflammatory activity of chondroitin sulphate: New functions from an old natural macromolecule. Inflammopharmacology 2011, 19, 299–306. [Google Scholar] [CrossRef]
- Borsig, L.; Wang, L.; Cavalcante, M.C.M.; Cardilo-Reis, L.; Ferreira, P.L.; Mourão, P.A.S.; Esko, J.D.; Pavão, M.S.G. Selectin Blocking Activity of a Fucosylated Chondroitin Sulfate Glycosaminoglycan from Sea Cucumber. J. Biol. Chem. 2007, 282, 14984–14991. [Google Scholar] [CrossRef] [Green Version]
- Asimakopoulou, A.P.; Theocharis, A.D.; Tzanakakis, G.N.; Karamanos, N.K. The Biological Role of Chondroitin Sulfate in Cancer and Chondroitin-based Anticancer Agents. In Vivo 2008, 390, 385–389. [Google Scholar]
- Thelin, M.A.; Svensson, K.J.; Shi, X.; Bagher, M.; Axelsson, J.; Isinger-ekstrand, A.; Van Kuppevelt, T.H.; Johansson, J.; Nilbert, M.; Zaia, J.; et al. Dermatan Sulfate Is Involved in the Tumorigenic Properties of Esophagus Squamous Cell Carcinoma. Cancer Res. 2012, 72, 1943–1953. [Google Scholar] [CrossRef]
- Kato, D.; Era, S.; Watanabe, I.; Arihara, M.; Sugiura, N.; Kimata, K.; Suzuki, Y.; Morita, K.; Hidari, K.I.P.J.; Suzuki, T. Antiviral activity of chondroitin sulphate E targeting dengue virus envelope protein. Antivir. Res. 2010, 88, 236–243. [Google Scholar] [CrossRef]
- Bergefall, K.; Trybala, E.; Johansson, M.; Uyama, T.; Naito, S.; Yamada, S.; Kitagawa, H.; Sugahara, K.; Bergstro, T. Chondroitin Sulfate Characterized by the E-disaccharide Unit Is a Potent Inhibitor of Herpes Simplex Virus Infectivity and Provides the Virus Binding Sites on gro2C Cells. J. Biol. Chem. 2005, 280, 32193–32199. [Google Scholar] [CrossRef] [Green Version]
- Lian, W.; Wu, M.; Huang, N.; Gao, N.; Xiao, C.; Li, Z.; Zhang, Z.; Zheng, Y.; Peng, W.; Zhao, J. Anti-HIV-1 activity and structure-activity-relationship study of a fucosylated glycosaminoglycan from an echinoderm by targeting the conserved CD4 induced epitope. Biochim. Biophys. Acta. 2013, 1830, 4681–4691. [Google Scholar] [CrossRef]
- Huang, N.; Wu, M.; Zheng, C.; Zhu, L.; Zhao, J.; Zheng, Y. The depolymerized fucosylated chondroitin sulfate from sea cucumber potently inhibits HIV replication via interfering with virus entry. Carbohyd. Res. 2013, 380, 64–69. [Google Scholar] [CrossRef]
- Calamia, V.; Mateos, J.; Lourido, L.; Rocha, B.; Montell, E.; Vergés, J.; Blanco, F.J. Anti-angiogenic action of chondroitin sulfate: A novel target for osteoarthritis therapy. Abstr. Osteoarthr. Cartil. 2013, 21, S271. [Google Scholar] [CrossRef]
- Tapon-Bretaudière, J.; Chabut, D.; Zierer, M.; Matou, S.; Helley, D.; Bros, A.; Mourão, P.A.S.; Fischer, A. A Fucosylated Chondroitin Sulfate from Echinoderm Modulates in Vitro Fibroblast Growth Factor 2—Dependent Angiogenesis. Mol. Cancer Res. 2002, 1, 96–102. [Google Scholar]
- Bastos, M.F.; Albrecht, L.; Kozlowski, E.O.; Lopes, S.C.; Blanco, Y.C.; Carlos, B.C.; Castiñeiras, C.; Vicente, C.P.; Werneck, C.C.; Wunderlich, G.; et al. Fucosylated chondroitin sulfate inhibits Plasmodium falciparum cytoadhesion and merozoite invasion. Antimicrob Agents Chemother. 2014, 58, 1862–1871. [Google Scholar] [CrossRef]
- Minamiguchi, K.G.; Kitazato, K.T.; Sasaki, E.; Nagase, H.; Kitazato, K. The Anticoagulant and Hemorrhagic Effects of DHG, a New Depolymerized Holothurian Glycosaminoglycan, on Experimental Hemodialysis in Dogs. Thromb. Haemost. 1997, 77, 1148–1153. [Google Scholar] [CrossRef]
- Tapon-Bretaudière, J.; Drouet, B.; Matou, S.; Mourão, A.S.; Bros, A.; Letourneur, D.; Fischer, A.M. Modulation of Vascular Human Endothelial and Rat Smooth Muscle Cell Growth by a Fucosylated Chondroitin Sulfate from Echinoderm. Thromb. Haemost. 2000, 84, 332–337. [Google Scholar] [CrossRef]
- Melo-filho, N.M.; Belmiro, C.L.; Gonçalves, R.G.; Takiya, C.M.; Leite, M.; Pavão, M.S.G.; Mourão, P.A.S. Fucosylated chondroitin sulfate attenuates renal fibrosis in animals submitted to unilateral ureteral obstruction: A P-selectin-mediated even? Am. J. Physiol. Renal Physiol. 2010, 299, F1299–F1307. [Google Scholar] [CrossRef]
- Hu, S.; Chang, Y.; Wang, J.; Xue, C.; Shi, D.; Xu, H.; Wang, Y. Fucosylated chondroitin sulfate from Acaudina molpadioides improves hyperglycemia via activation of PKB/GLUT4 signaling in skeletal muscle of insulin resistant mice. Food Funct. 2013, 4, 1639–1646. [Google Scholar] [CrossRef]
- Gomes, C.L.; Leão, C.L.; Venturotti, C.; Barreira, A.L.; Guimarães, G.; Fonseca, R.J.; Fortunato, R.S.; Mourão, P.A.; Delgado, A.G.; Takiya, C.M.; et al. The Protective Role of Fucosylated Chondroitin Sulfate, a Distinct Glycosaminoglycan, in a Murine Model of Streptozotocin-Induced Diabetic Nephropathy. PLoS ONE 2014, 9, e106929. [Google Scholar] [CrossRef]
- Hart, G.W.; Copeland, R.J. Glycomics Hits the Big Time. Cell 2010, 143, 672–676. [Google Scholar] [CrossRef] [Green Version]
- Woods Group GLYCAM Web. Complex Carbohydrate Research Center, University of Georgia, Athens, GA. Available online: http://glycam.org (accessed on 30 May 2019).
- Nandini, C.D.; Sugahara, K. Role of the Sulfation Pattern of Chondroitin Sulfate in its Biological Activities and in the Binding of Growth Factors. Adv. Pharmacol. 2006, 53, 253–279. [Google Scholar] [CrossRef]
- Yamada, S.; Sugahara, K.; Özbek, S. Evolution of glycosaminoglycans: Comparative biochemical study. Commun. Integr. Biol. 2011, 4, 150–158. [Google Scholar] [CrossRef]
- Cássaro, C.M.; Dietrich, C.P. Distribution of sulfated mucopolysaccharides in invertebrates. J. Biol. Chem. 1977, 252, 2254–2261. [Google Scholar]
- Medeiros, G.F.; Mendes, A.; Castro, R.A.; Baú, E.C.; Nader, H.B.; Dietrich, C.P. Distribution of sulfated glycosaminoglycans in the animal kingdom: Widespread occurrence of heparin-like compounds in invertebrates. Biochim. Biophys. Acta 2000, 1475, 287–294. [Google Scholar] [CrossRef]
- Sampaio, L.O.; Nader, H.B. Emergence and structural characteristics of chondroitin sulfates in the animal kingdom. Adv. Pharmacol. 2006, 53, 233–251. [Google Scholar] [CrossRef]
- Kinoshita, A.; Yamada, S.; Haslam, S.M.; Morris, H.R.; Dell, A.; Sugahara, K. Isolation and Structural Determination of Novel Sulfated Hexasaccharides from Squid Cartilage Chondroitin Sulfate E That Exhibits Neuroregulatory Activities. Biochemistry 2001, 40, 12654–12665. [Google Scholar] [CrossRef]
- Vázquez, J.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González, M.; Murado Anxo, M. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs. 2013, 11, 747–774. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, R.S.; Brito, A.S.; Palhares, L.C.G.F.; Lima, M.A.; Cavalheiro, R.P.; Nader, H.B.; Sassaki, G.L.; Chavante, S.F. 2,3-Di-O-sulfo glucuronic acid: An unmodified and unusual residue in a highly sulfated chondroitin sulfate from Litopenaeus vannamei. Carbohydr. Polym. 2018, 183, 192–200. [Google Scholar] [CrossRef]
- Caterson, B.; Mahmoodian, F.; Sorrell, J.M.; Tim, E.; Bayliss, M.T.; Carney, S.L.; Ratcliffe, A.; Muir, H. Modulation of native chondroitin sulphate structure in tissue development and in disease. J. Cell Sci. 1990, 97 Pt 3, 411–417. [Google Scholar]
- Farrugia, B.L.; Lord, M.S.; Whitelock, J.M.; Melrose, J. Harnessing chondroitin sulphate in composite scaffolds to direct progenitor and stem cell function for tissue repair. Biomate. Sci. 2018, 6, 947–957. [Google Scholar] [CrossRef]
- Sugahara, K.; Mikami, T. Chondroitin/dermatan sulfate in the central nervous system. Curr. Opin. Struct. Biol. 2007, 17, 536–545. [Google Scholar] [CrossRef]
- Nadanaka, S.; Clement, A.; Masayama, K.; Faissner, A.; Sugahara, K.; Biol, M.J.C. Characteristic Hexasaccharide Sequences in Octasaccharides Derived from Shark Cartilage Chondroitin Sulfate D with a Neurite Outgrowth Promoting Activity. J. Biol. Chem. 1998, 273, 3296–3307. [Google Scholar] [CrossRef] [Green Version]
- Shida, M.; Mikami, T.; Tamura, J.; Kitagawa, H. A characteristic chondroitin sulfate trisaccharide unit with a sulfated fucose branch exhibits neurite outgrowth-promoting activity: Novel biological roles of fucosylated chondroitin sulfates isolated from the sea cucumber Apostichopus JPN. Biochem. Biophys. Res. Commun. 2017, 487, 1–6. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Farran, A.; Montell, E.; Vergés, J.; Pelletier, J. Discrepancies in Composition and Biological Effects of Different Formulations of Chondroitin Sulfate. Molecules 2015, 20, 4277–4289. [Google Scholar] [CrossRef] [Green Version]
- Bishnoi, M.; Jain, A.; Hurkat, P.; Jain, S.K. Chondroitin sulphate: A focus on osteoarthritis. Glycoconj. J. 2016, 52, 693–705. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Clegg, D.O. Potential effects of chondroitin sulfate on joint swelling: A GAIT report. Osteoarthr. Cartil. 2008, 16, 22–24. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Martel-Pelletier, J.; Monfort, J.; Möller, I.; Castillo, J.R.; Arden, N.; Berenbaum, F.; Blanco, F.J.; Conaghan, P.G.; Doménech, G.; et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: A multicentre randomised, double-blind, non-inferiority trial, versus. Celecoxib 2016, 75, 37–44. [Google Scholar] [CrossRef]
- Población, C.A.; Michelacci, Y.M. Structural differences of dermatan sulfates from different origins. Carbohydr. Res. 1986, 147, 87–100. [Google Scholar] [CrossRef]
- Sim, J.; Im, A.; Mock, S.; Jin, H.; Ho, J.; Shik, Y. Evaluation of chondroitin sulfate in shark cartilage powder as a dietary supplement: Raw materials and finished products. Food Chem. 2007, 101, 532–539. [Google Scholar] [CrossRef]
- Falshaw, R.; Hubl, U.; Ofman, D.; Slim, G.C.; Tariq, M.A.; Watt, D.K.; Yorke, S.C. Comparison of the glycosaminoglycans isolated from the skin and head cartilage of Gould’s arrow squid (Nototodarus gouldi). Carbohydr. Polym. 2000, 41, 357–364. [Google Scholar] [CrossRef]
- Higashi, K.; Okamoto, Y.; Mukuno, A.; Wakai, J.; Hosoyama, S.; Linhardt, R.J.; Toida, T. Functional chondroitin sulfate from Enteroctopus dofleini containing a 3-O-sulfo glucuronic acid residue. Carbohydr. Polym. 2015, 134, 557–565. [Google Scholar] [CrossRef]
- Sugahara, K.; Tanaka, Y.; Yamada, S.; Seno, N.; Kitagawa, H.; Haslam, S.M.; Morris, H.R.; Dell, A. Novel Sulfated Oligosaccharides Containing 3-O-Sulfated Glucuronic Acid from King Crab Cartilage Chondroitin Sulfate K. Unexpected degradation by chondroitinase ABC. J. Biol. Chem. 1996, 271, 26745–26754. [Google Scholar] [CrossRef]
- Garnjanagoonchorn, W.; Wongekalak, L.; Engkagul, A. Determination of chondroitin sulfate from different sources of cartilage. Chem. Eng. Process. Process. Intensif. 2007, 46, 465–471. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Linhardt, R.J.; Liu, H.; Linhardt, R.J. Lessons learned from the contamination of heparin. Nat. Prod. Rep. 2009, 26, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Pomin, V.H.; Piquet, A.A.; Pereira, M.S.; Mourão, P.A.S. Residual keratan sulfate in chondroitin sulfate formulations for oral administration. Carbohydr. Polym. 2012, 90, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.O.; Kim, M.; Woo, M.; Baek, J.; Kang, K.; Kim, S.H.; Roh, S.S.; Park, C.H.; Jeong, K.S.; Noh, J.S. Chondroitin Sulfate-Rich Extract of Skate Cartilage Attenuates Lipopolysaccharide-Induced Liver Damage in Mice. Mar. Drugs 2017, 15, 178. [Google Scholar] [CrossRef]
- Kawashima, H.; Atarashi, K.; Hirose, M.; Hirose, J.; Yamada, S.; Sugahara, K.; Miyasaka, M. Oversulfated chondroitin/dermatan sulfates containing GlcAβ1/IdoAα1-3GalNAc(4,6-O-disulfate) interact with L- and P-selectin and chemokines. J. Biol. Chem. 2002, 277, 12921–12930. [Google Scholar] [CrossRef]
- Yip, G.W.; Smollich, M.; Go, M. Therapeutic value of glycosaminoglycans in cancer. Mol. Cancer Ther. 2006, 5, 2139–2149. [Google Scholar] [CrossRef]
- Cooney, C.A.; Jousheghany, F.; Yao-borengasser, A.; Phanavanh, B.; Gomes, T.; Kieber-emmons, A.M.; Siegel, E.R.; Suva, L.J.; Ferrone, S.; Kieber-emmons, T. Chondroitin sulfates play a major role in breast cancer metastasis: A role for CSPG4 and CHST11 gene expression in forming surface P-selectin ligands in aggressive breast cancer cells. Breast Cancer Res. 2011, 13, R58. [Google Scholar] [CrossRef]
- Razin, E.; Stevens, R.L.; Akiyama, F.; Schmid, K.; Austen, K.F. Culture from Mouse Bone Marrow of a Subclass of Mast Cells Possessing a Distinct Chondroitin Sulfate Proteoglycan with Glycosaminoglycans Rich in in N-acetylgalactosamine-4,6-disulfate. J. Biol. Chem. 1982, 257, 7229–7236. [Google Scholar]
- Stevens, R.L.; Fox, C.C.; Lichtenstein, L.M.; Austen, K.F. Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc. Natl. Acad. Sci. USA 1988, 85, 2284–2287. [Google Scholar] [CrossRef]
- Yamada, S.; Sugahara, K. Potential Therapeutic Application of Chondroitin Sulfate/Dermatan Sulfate. Curr. Drug Discov. Technol. 2008, 5, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Hileman, R.E.; Fromm, J.R.; Weiler, J.M.; Linhardt, R.J. Glycosaminoglycan-protein interactions: Definition of consensus sites in glycosaminoglycan binding proteins. BioEssays 1998, 20, 156–167. [Google Scholar] [CrossRef]
- Kjellén, L.; Lindahl, U. Specificity of glycosaminoglycan—Protein interactions. Curr. Opin. Struct. Biol. 2018, 50, 101–108. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Suda, H.; Tsuzuike, N.; Seto, K.; Seki, M.; Yamaguchi, Y.; Hasegawa, K.; Takahashi, N.; Yamamoto, S.; Gejyo, F.; et al. Glycosaminoglycan and proteoglycan inhibit the depolymerization of β2-microglobulin amyloid fibrils in vitro. Kindey Int. 2003, 64, 1080–1088. [Google Scholar] [CrossRef]
- Kimura, K.; Matsubara, H.; Sogoh, S.; Kita, Y.; Sakata, T.; Nishitani, Y.; Watanabe, S.; Hamaoka, T.; Fujiwara, H. Role of glycosaminoglycans in the regulation of T cell proliferation induced by thymic stroma-derived T cell growth factor. J. Immunol. 1991, 146, 2618–2624. [Google Scholar]
- Vallières, M.; Souich, P. Modulation of inflammation by chondroitin sulfate. Osteoarthr. Cartil. 2010, 18, 18–23. [Google Scholar] [CrossRef]
- Du Souich, P.; García, A.G.; Vergés, J.; Montell, E. Immunomodulatory and anti-inflammatory effects of chondroitin sulphate. J. Cell. Mol. Med. 2009, 13, 1451–1463. [Google Scholar] [CrossRef]
- Linares, P.M.; Chaparro, M.; Algaba, A.; Román, M.; Moreno Arza, I.; Abad Santos, F.; Ochoa, D.; Guerra, I.; Bermejo, F.; Gisbert, J.P. Effect of Chondroitin Sulphate on Pro-Inflammatory Mediators and Disease Activity in Patients with Inflammatory Bowel Disease. Digestion 2015, 92, 203–210. [Google Scholar] [CrossRef]
- Schön, M.P. Inhibitors of selectin functions in the treatment of inflammatory skin disorders. Ther. Clin. Risk Manag. 2005, 1, 201–208. [Google Scholar]
- Deshauer, C.; Morgan, A.M.; Prestegard, J.H.; Wang, X.; Deshauer, C.; Morgan, A.M.; Ryan, E.O.; Handel, T.M.; Prestegard, J.H.; Wang, X. Interactions of the Chemokine CCL5/RANTES with Medium-Sized Chondroitin Sulfate Ligands. Cell 2015, 23, 1066–1077. [Google Scholar] [CrossRef] [Green Version]
- Willis, C.M.; Klu, M. Chondroitin Sulfate-E Is a Negative Regulator of a Pro- Tumorigenic Wnt/Beta-Catenin-Collagen 1 Axis in Breast Cancer Cells. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Barthel, S.R.; Gavino, J.D.; Descheny, L.; Dimitroff, C.J. Targeting selectins and selectin ligands in inflammation and cancer. J. Expert Opin. Ther. Targets. 2007, 11, 1473–1491. [Google Scholar] [CrossRef]
- Kim, Y.J.; Borsig, L.; Han, H.; Varki, N.M.; Varki, A. Distinct Selectin Ligands on Colon Carcinoma Mucins Can Mediate Pathological Interactions among Platelets, Leukocytes, and Endothelium. Am. J. Pathol. 1999, 155, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Laabs, T.; Carulli, D.; Geller, H.M.; Fawcett, J.W. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr. Opin. Neurobiol. 2005, 15, 116–120. [Google Scholar] [CrossRef]
- Rauvala, H.; Paveliev, M.; Kuja-panula, J.; Kulesskaya, N. Inhibition and enhancement of neural regeneration by chondroitin sulfate proteoglycans. Neural Regen. Res. 2017, 12, 687–692. [Google Scholar] [CrossRef]
- Siebert, J.R.; Steencken, A.C.; Osterhout, D.J. Chondroitin Sulfate Proteoglycans in the Nervous System: Inhibitors to Repair. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Ito, Z.; Sakamoto, K.; Imagama, S.; Matsuyama, Y.; Zhang, H.; Hirano, K.; Ando, K.; Yamashita, T.; Ishiguro, N.; Kadomatsu, K. N -Acetylglucosamine 6- O -Sulfotransferase-1-Deficient Mice Show Better Functional Recovery after Spinal Cord Injury. J. Neurosci. 2010, 30, 5937–5947. [Google Scholar] [CrossRef]
- Imagama, S.; Sakamoto, K.; Tauchi, R.; Shinjo, R.; Ohgomori, T.; Ito, Z.; Zhang, H.; Nishida, Y.; Asami, N.; Takeshita, S.; et al. Keratan Sulfate Restricts Neural Plasticity after Spinal Cord Injury. J. Neurosci. 2011, 31, 17091–17102. [Google Scholar] [CrossRef] [Green Version]
- Ueoka, C.; Kaneda, N.; Okazaki, I.; Nadanaka, S.; Muramatsu, T.; Sugahara, K. Neuronal Cell Adhesion, mediated by the Heparin-binding Neuroregulatory Factor Midkine, Is Specifically Inhibited by Chondroitin Sulfate E. J. Biol. Chem. 2000, 275, 37407–37413. [Google Scholar] [CrossRef]
- Maeda, N.; Fukazawa, N.; Hata, T. The Binding of Chondroitin Sulfate to Pleiotrophin/Heparin- binding Growth-associated Molecule Is Regulated by Chain Length and Oversulfated Structures. J. Biol. Chem. 2005, 281, 4894–4902. [Google Scholar] [CrossRef]
- Maeda, N.; He, J.; Yajima, Y.; Mikami, T.; Sugahara, K.; Yabe, T. Heterogeneity of the Chondroitin Sulfate Portion of Phosphacan/6B4 Proteoglycan Regulates Its Binding Affinity for Pleiotrophin/Heparin Binding Growth-associated Molecule. J. Biol. Chem. 2003, 278, 35805–35811. [Google Scholar] [CrossRef] [Green Version]
- Maeda, N.; Nishiwaki, T.; Shintani, T.; Hamanaka, H.; Noda, M. 6B4 Proteoglycan/Phosphacan, an Extracellular Variant of Receptor-like Protein-tyrosine Phosphatase ζ/RPTP β, Binds Pleiotrophin/Heparin-binding Growth-associated Molecule. J. Biol. Chem. 1996, 271, 21446–21452. [Google Scholar] [CrossRef]
- Deepa, S.S.; Umehara, Y.; Higashiyama, S.; Itoh, N.; Sugahara, K. Specific Molecular Interactions of Oversulfated Chondroitin Sulfate E with Various Heparin-binding Growth Factors. J. Biol. Chem. 2002, 277, 43707–43716. [Google Scholar] [CrossRef] [Green Version]
- Rani, A.; Patel, S.; Goyal, A. Chondroitin Sulfate (CS) Lyases: Structure, Function and Application in Therapeutics. Curr. Protein Pept. Sci. 2018, 19, 22–33. [Google Scholar] [CrossRef]
- Lin, Y.; Li, L.; Zhang, F.; Linhardt, R.J. Borrelia burgdorferi glycosaminoglycan-binding proteins: A potential target for new therapeutics against Lyme disease. Microbiology 2017, 163, 1759–1766. [Google Scholar] [CrossRef]
- Chaffee, K.M.A.E. Mucopolysaccharides of Skin. J. Biol. Chem. 1941, 138, 491–499. [Google Scholar]
- Singla, S.K. Dermatan sulphate structure biosynthesis and functions. Biochem. Educ. 1988, 16, 156–157. [Google Scholar] [CrossRef]
- Trowbridge, J.M.; Gallo, R.L. Dermatan sulfate: New functions from an old glycosaminoglycan. Glycobiology 2002, 12, 117R–125R. [Google Scholar] [CrossRef]
- Osborne, S.A.; Daniel, R.A.; Desilva, K.; Seymour, R.B. Antithrombin activity and disaccharide composition of dermatan sulfate from different bovine tissues. Glycobiology 2008, 18, 225–234. [Google Scholar] [CrossRef]
- Malavaki, C.; Mizumoto, S.; Karamanos, N.; Sugahara, K. Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connect. Tissue Res. 2008, 49, 133–139. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Pomin, V. The Sea as a Rich Source of Structurally Unique Glycosaminoglycans and Mimetics. Microorganisms 2017, 5, 51. [Google Scholar] [CrossRef]
- Bossennec, V.; Petitou, M.; Perly, B. 1H-n.m.r. investigation of naturally occurring and chemically oversulphated dermatan sulphates. Identification of minor monosaccharide residues. Biochem. J. 1990, 267, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Pavão, M.S.; Aiello, K.R.; Werneck, C.C.; Silva, L.C.F.; Valente, A.P.; Mulloy, B.; Colwell, N.S.; Tollefsen, D.M.; Mourão, P.A.S. Highly sulfated dermatan sulfates from Ascidians. Structure versus anticoagulant activity of these glycosaminoglycans. J. Biol. Chem. 1998, 273, 27848–27857. [Google Scholar] [CrossRef]
- Pavão, M.; Mourão, P.; Mulloy, B.; Tollefsen, D. A unique dermatan sulfate-like glycosaminoglycan from Ascidian: Its structure and the effect of its unusual sulfation pattern on anticoagulant activity. J. Biol. Chem. 1995, 270, 31027–31036. [Google Scholar] [CrossRef]
- Pavão, M.S.G. Structure and anticoagulant properties of sulfated glycosaminoglycans from primitive Chordates. An. Acad. Bras. Cienc. 2002, 74, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Pomin, V.H. A Dilemma in the Glycosaminoglycan-Based Therapy: Synthetic or Naturally Unique Molecules? Med. Res. Rev. 2015, 35, 1195–1219. [Google Scholar] [CrossRef]
- Sanderson, P.N.; Huckerby, T.N.; Nieduszynski, I.A. Chondroitinase ABC digestion of dermatan sulphate. N.m.r. spectroscopic characterization of the oligo- and poly-saccharides. Biochem. J. 1989, 257, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Pavão, M.S.G.; Rodrigues, M.A.; Mourão, P.A.S. Acidic polysaccharides of the ascidian Styela plicata. Biosynthetic studies on the sulfated l-galactans of the tunic, and preliminary characterization of a dermatan sulfate-like polymer in body tissues. BBA Gen. Subj. 1994, 1199, 229–237. [Google Scholar] [CrossRef]
- Anno, K.; Seno, N.; Mathews, M.B.; Yamagata, T.; Suzukic, S. A new dermatan polysulfate, chondroitin sulfate H, from hagfish notochord. BBA Gen. Subj. 1971, 237, 173–177. [Google Scholar] [CrossRef]
- Seno, N.; Fumiko, A.; Anno, K. A novel dermatan polysulfate from hagfish skin, containing trisulfated disaccharide residues. BBA Gen. Subj. 1972, 264, 229–233. [Google Scholar] [CrossRef]
- Sugahara, K.; Yamada, S. Structure and Function Unique Sulfation of Oversulfated Chondroitin Patterns and Neuroregulatory Sulfate Variants. Trends Glycosci. Glyc. 2000, 12, 321–349. [Google Scholar] [CrossRef]
- Casu, B.; Guerrini, M.; Torri, G. Structural and conformational aspects of the anticoagulant and anti-thrombotic activity of heparin and dermatan sulfate. Curr. Pharm. Des. 2004, 10, 939–949. [Google Scholar] [CrossRef]
- Fernandez, F.; Van Ryn, J.; Ofosu, F.A.; Hirsh, J.; Buchanan, M.R. The haemorrhagic and antithrombotic effects of dermatan sulphate. Br. J. Haematol. 1986, 64, 309–317. [Google Scholar] [CrossRef]
- Linhardt, R.J.; Hileman, R.E. Dermatan sulfate as a potential therapeutic agent. Gen. Pharmacol. 1995, 26, 443–451. [Google Scholar] [CrossRef]
- Buchanan, M.R.; Brister, S.J. Anticoagulant and antithrombin effects of intimatan, a heparin cofactor II agonist. Thromb. Res. 2000, 99, 603–612. [Google Scholar] [CrossRef]
- Buchanan, M.R.; Maclean, G.A.; Brister, S.J. Selective and sustained inhibition of surface-bound thrombin activity by intimatan/heparin cofactor II and its relevance to assessing systemic anticoagulation in vivo, ex vivo and in vitro. Thromb. Haemost. 2001, 86, 909–913. [Google Scholar]
- Hennan, J.K.; Hong, T.T.; Shergill, A.K.; Driscoll, E.M.; Cardin, A.D.; Lucchesi, B.R. Intimatan prevents arterial and venous thrombosis in a canine model of deep vessel wall injury. J. Pharmacol. Exp. Ther. 2002, 301, 1151–1156. [Google Scholar] [CrossRef]
- Hong, T.T.; White, A.J.; Lucchesi, B.R. Dermatan disulfate (Intimatan) prevents complement-mediated myocardial injury in the human-plasma-perfused rabbit heart. Int. Immunopharmacol. 2005, 5, 381–391. [Google Scholar] [CrossRef]
- Hong, T.T.; Van Gorp, C.L.; Cardin, A.D.; Lucchesi, B.R. Intimatan (dermatan 4,6-O-disulfate) prevents rethrombosis after successful thrombolysis in the canine model of deep vessel wall injury. Thromb. Res. 2006, 117, 333–342. [Google Scholar] [CrossRef]
- Tanaka, K.A.; Szlam, F.; Vinten-Johansen, J.; Cardin, A.D.; Levy, J.H. Effects of antithrombin and heparin cofactor II levels on anticoagulation with Intimatan. Thromb. Haemost. 2005, 94, 808–813. [Google Scholar] [CrossRef]
- Mascellani, G.; Liverani, L.; Prete, A.; Guppola, P.A.; Bergonzini, G.; Bianchini, P. Relative influence of different disulphate disaccharide clusters on the HCII-mediated inhibition of thrombin by dermatan sulphates of different origins. Thromb. Res. 1994, 74, 605–615. [Google Scholar] [CrossRef]
- Mascellani, G.; Liverani, L.; Prete, A.; Bergonzini, G.; Bianchini, P.; Torri, G.; Bisio, A.; Guerrini, M.; Casu, B. Quantitation of dermatan sulfate active site for heparin cofactor II by 1H nuclear magnetic resonance spectroscopy. Anal. Biochem. 1994, 223, 135–141. [Google Scholar] [CrossRef]
- Maimone, M.M.; Tollefsen, D.M. Structure of a dermatan sulfate hexasaccharide that binds to heparin cofactor II with high affinity. J. Biol. Chem. 1990, 265, 18263–18271. [Google Scholar]
- Basten, J.E.M.; Van Boeckel, C.A.A.; Jaurand, G.; Petitou, M.; Soijker, N.M.; Westerduin, P. Synthesis of a dermatan sulphate-like hexasaccharide with a “non-glycosamino” glycan structure. Bioorg. Med. Chem. Lett. 1994, 4, 893–898. [Google Scholar] [CrossRef]
- Yang, H.O.; Gunay, N.S.; Toida, T.; Kuberan, B.; Yu, G.; Kim, Y.S.; Linhardt, R.J. Preparation and structural determination of dermatan sulfate-derived oligosaccharides. Glycobiology 2000, 10, 1033–1039. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Lassepas, M.; Quinones, M.R. Heparin therapy for stroke Hemorrhagic complications and risk factors for intracerebral hemorrhage. Neurology 1984, 34, 114. [Google Scholar] [CrossRef]
- Hirsh, J. Heparin induced bleeding. Nouv. Rev. Fr. Hematol. 1984, 26, 261–266. [Google Scholar]
- Qin, X.; Zhang, B. Cerebral Hemorrhage Complicating Heparin—Induced Thrombocytopenia after Percutaneous Coronary Intervention: A Clinical Dilemma of Medical Treatment. Chin. Med. J. 2015, 128, 1561–1562. [Google Scholar] [CrossRef]
- Babikian, V.L.; Kase, C.S.; Pessin, M.S.; Norrving, B.; Gorelick, P.B. Intracerebral Hemorrhage in Stroke Patients Anticoagulated with Heparin. Stroke 1989, 20, 1500–1503. [Google Scholar] [CrossRef]
- Gianese, F.; Nurmohamed, M.; Imbimbo, B.; Buller, H.; Berckmans, R.; Ten Cate, J. The pharmacokinetics and pharmacodynamics of dermatan sulphate MF701 during haemodialysis for chronic renal failure. Br. J. Clin. Pharmacol. 1993, 35, 335–339. [Google Scholar] [CrossRef]
- Tollefsens, D.M.; Peacock, M.E.; Monafo, W.J. Molecular Size of Dermatan Sulfate Oligosaccharides Required to Bind and Activate Heparin Cofactor II. J. Biol. Chem. 1986, 261, 8854–8858. [Google Scholar]
- Vicente, C.P.; He, L.; Pavão, M.S.G.; Tollefsen, D.M. Antithrombotic activity of dermatan sulfate in heparin cofactor II-deficient mice. Blood 2004, 104, 3965–3970. [Google Scholar] [CrossRef]
- Linhardt, R.J.; Desai, U.R.; Liu, J.; Pervin, A.; Hoppensteadt, D.; Fareed, J. Low molecular weight dermatan sulfate as an antithrombotic agent structure-activity relationship studies. Biochem. Pharmacol. 1994, 47, 1241–1252. [Google Scholar] [CrossRef]
- Linhardt, R.J.; Al-Hakim, A.; Liu, J.; Hoppensteadt, D.; Mascellani, G.; Bianchini, P.; Fareed, J. Structural features of dermatan sulfates and their relationship to anticoagulant and antithrombotic activities. Biochem. Pharmacol. 1991, 42, 1609–1619. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Tollefsen, D.M.; Sadler, J.E. Heparin cofactor II is regulated allosterically and not primarily by template effects. Studies with mutant thrombins and glycosaminoglycans. J. Biol. Chem. 1994, 269, 32747–32751. [Google Scholar]
- Plichta, J.K.; Radek, K.A. Sugar-coating wound repair: A review of FGF-10 and dermatan sulfate in wound healing and their potential application in burn wounds. J. Burn Care Res. 2012, 33, 299–310. [Google Scholar] [CrossRef]
- Penc, S.F.; Pomahac, B.; Winkler, T.; Dorschner, R.A.; Eriksson, E.; Herndon, M.; Gallo, R.L. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J. Biol. Chem. 1998, 273, 28116–28121. [Google Scholar] [CrossRef]
- Radek, K.A.; Taylor, K.R.; Gallo, R.L. FGF-10 and specific structural elements of dermatan sulfate size and sulfation promote maximal keratinocyte migration and cellular proliferation. Wound Repair Regen. 2009, 17, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Trowbridge, J.M.; Rudisill, J.A.; Ron, D.; Gallo, R.L. Dermatan sulfate binds and potentiates activity of keratinocyte growth factor (FGF-7). J. Biol. Chem. 2002, 277, 42815–42820. [Google Scholar] [CrossRef]
- Priglinger, U.; Geiger, M.; Bielek, E.; Vanyek, E.; Binder, B.R. Binding of urinary protein C inhibitor to cultured human epithelial kidney tumor cells (TCL-598). The role of glycosaminoglycans present on the luminal cell surface. J. Biol. Chem. 1994, 269, 14705–14710. [Google Scholar]
- Iozzo, R.V. The family of the small leucine-rich proteoglycans: Key regulators of matrix assembly and cellular growth. Crit. Rev. Biochem. Mol. Biol. 1997, 32, 141–174. [Google Scholar] [CrossRef]
- Tumova, S.; Woods, A.; Couchman, J.R. Heparan sulfate chains from glypican and syndecans bind the Hep II domain of fibronectin similarly despite minor structural differences. J. Biol. Chem. 2000, 275, 9410–9417. [Google Scholar] [CrossRef]
- Jungmann, O.; Nikolovska, K.; Stock, C.; Schulz, J.; Eckes, B.; Owens, R.T.; Iozzo, R.V.; Seidler, D.G.; Riethmu, C. The dermatan sulfate proteoglycan decorin modulates α2β1 integrin and the vimentin intermediate filament system during collagen synthesis. PLoS ONE 2012, 7, e50809. [Google Scholar] [CrossRef]
- Seo, E.S.; Blaum, S.; Vargues, T.; De Cecco, M.; Deakin, J.A.; Lyon, M.; Barran, P.E.; Campopiano, D.J.; Uhrín, D. Interaction of Human β -Defensin 2 (HBD2) with Glycosaminoglycans. Biochemistry 2010, 2, 10486–10495. [Google Scholar] [CrossRef]
- Brooks, B.; Briggs, D.M.; Eastmond, N.C.; Fernig, D.G.; Coleman, J.W. Presentation of IFN-γ to Nitric Oxide-Producing Cells: A Novel Function for Mast Cells. J. Immunol. 2000, 164, 573–579. [Google Scholar] [CrossRef]
- Hildebrand, A.; Romarís, M.; Rasmussen, L.M.; Heinegård, D.; Twardzik, D.R.; Border, W.A.; Ruoslahti, E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem. J. 1994, 302, 527–534. [Google Scholar] [CrossRef]
- Belmiro, C.L.R.; Souza, H.S.P.; Elia, C.C.S.; Castelo-Branco, M.T.L.; Silva, F.R.; Machado, R.L.; Pavão, M.S.G. Biochemical and immunohistochemical analysis of glycosaminoglycans in inflamed and non-inflamed intestinal mucosa of patients with Crohn’s disease. Int. J. Colorectal Dis. 2005, 20, 295–304. [Google Scholar] [CrossRef]
- Belmiro, C.L.R.; Gonçalves, R.G.; Kozlowski, E.O.; Werneck, A.F.; Takyia, C.M.; Leite, M., Jr.; Pavão, M.S.G. Dermatan sulfate reduces monocyte chemoattractant protein 1 and TGF-β production, as well as macrophage recruitment and myofibroblast accumulation in mice with unilateral ureteral obstruction. Brazilian J. Med. Biol. Res. 2011, 44, 624–633. [Google Scholar] [CrossRef]
- Gruber, S.; Frings, K.; Kuess, P.; Dörr, W. Protective effects of systemic dermatan sulfate treatment in apreclinical model of radiation-induced oral mucositis. Strahlenthe. Onkol. 2018, 194, 675–685. [Google Scholar] [CrossRef]
- Gruber, S.; Arnold, M.; Cini, N.; Gernedl, V.; Hetzendorfer, S.; Kowald, L.M.; Kuess, P.; Mayer, J.; Morava, S.; Pfaffinger, S.; et al. Radioprotective effects of dermatan sulfate in a preclinical model of oral mucositis—targeting inflammation, hypoxia and junction proteins without stimulating proliferation. Int. J. Mol. Sci. 2018, 19, 1684. [Google Scholar] [CrossRef]
- Afratis, N.; Gialeli, C.; Nikitovic, D.; Tsegenidis, T.; Karousou, E.; Tzanakakis, G.N.; Karamanos, N.K. Glycosaminoglycans: Key players in cancer cell biology and treatment. FEBS J. 2012, 279, 1177–1197. [Google Scholar] [CrossRef]
- Kozlowski, E.O.; Pavao, M.S.G.; Borsig, L. Ascidian dermatan sulfates attenuate metastasis, inflammation and thrombosis by inhibition of P-selectin. J. Thromb. Haemost. 2011, 9, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- Di Caro, A.; Perola, E.; Bartoloni, B.; Marzano, M.; Liverani, L.; Mascellani, G.; Benedetto, A.; Cellai, L. Fractions of chemically oversulphated galactosaminoglycan sulphates inhibit three enveloped viruses: Human immunodeficiency virus type 1, herpes simplex virus type 1 and human cytomegalovirus. Antivir. Chem. Chemother. 1999, 10, 33–38. [Google Scholar] [CrossRef]
- Nagase, B.H.; Enjyoji, K.; Minamiguchi, K.; Kitazato, K.T.; Kitazato, K.; Saito, H. Depolymerized holothurian glycosaminoglycan with novel anticoagulant actions: Antithrombin III- and heparin cofactor II-independent inhibition of factor X activation by factor IXa-factor VIIIa complex and heparin cofactor II-dependent inhibition of thrombin. Blood 1995, 85, 1527–1534. [Google Scholar]
- Wu, M.; Wen, D.; Gao, N.; Xiao, C.; Yang, L.; Xu, L.; Lian, W.; Peng, W.; Jiang, J.; Zhao, J. Anticoagulant and antithrombotic evaluation of native fucosylated chondroitin sulfates and their derivatives as selective inhibitors of intrinsic factor Xase. Eur. J. Med. Chem. 2015, 92, 257–269. [Google Scholar] [CrossRef]
- Lidholt, K.; Fjelstad, M. Biosynthesis of the Escherichia coli K4 Capsule Polysaccharide A parallel system for studies of glycosyltransferases in chondroitin formation. J. Biol. Chem. 1997, 272, 2682–2687. [Google Scholar] [CrossRef]
- Habuchi, O.; Sugiura, K.; Kawai, N.; Suzuki, S. Glucose Branches in Chondroitin Sulfates from Squid Cartilage. J. Biol. Chem. 1977, 252, 4570–4576. [Google Scholar]
- Higashi, K.; Takeda, K.; Mukuno, A.; Okamoto, Y.; Masuko, S.; Linhardt, R.J.; Toida, T. Identification of keratan sulfate disaccharide at C-3 position of glucuronate of chondroitin sulfate from Mactra chinensis. Biochem. J. 2016, 473, 4145–4158. [Google Scholar] [CrossRef]
- Lord, M.S.; Day, A.J.; Youssef, P.; Zhuo, L.; Watanabe, H.; Caterson, B.; Whitelock, J.M. Sulfation of the bikunin chondroitin sulfate chain determines heavy chain·hyaluronan complex formation. J. Biol. Chem. 2013, 288, 22930–22941. [Google Scholar] [CrossRef]
- Zhuo, L.; Hascall, V.C.; Kimata, K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J. Biol. Chem. 2004, 279, 38079–38082. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Nifantiev, N.E.; Usov, A.I. New insight on the structural diversity of holothurian fucosylated chondroitin sulfates. Pure Appl. Chem. 2019, 91, 1–7. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mulloy, B.; Mourão, P.A. Structure of a fucose-branched chondroitin sulfate from sea cucumber. Evidence for the presence of 3-O-sulfo-beta-d-glucuronosyl residues. J. Biol. Chem. 1991, 266, 13530–13536. [Google Scholar]
- Pomin, V.H. Medical Gains of Chondroitin Sulfate Upon Fucosylation. Curr. Med. Chem. 2015, 22, 4166–4176. [Google Scholar] [CrossRef]
- Fonseca, R.J.C.; Santos, G.R.C.; Mourão, P.A.S. Effects of polysaccharides enriched in 2,4-disulfated fucose units on coagulation, thrombosis and bleeding Practical and conceptual implications. Thromb. Haemost. 2009, 102, 829–836. [Google Scholar] [CrossRef]
- Chen, S.; Xue, C.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Wu, M.; Xu, S.; Zhao, J.; Kang, H.; Ding, H. Physicochemical characteristics and anticoagulant activities of low molecular weight fractions by free-radical depolymerization of a fucosylated chondroitin sulphate from sea cucumber Thelenata Ananas. Food Chem. 2010, 122, 716–723. [Google Scholar] [CrossRef]
- Wu, M.; Huang, R.; Wen, D.; Gao, N.; He, J.; Li, Z.; Zhao, J. Structure and effect of sulfated fucose branches on anticoagulant activity of the fucosylated chondroitin sulfate from sea cucumber Thelenata ananas. Carbohydr. Polym. 2012, 87, 862–868. [Google Scholar] [CrossRef]
- Kariya, Y.; Watabe, S.; Kyogashima, M.; Ishihara, M.; Ishii, T. Structure of fucose branches in the glycosaminoglycan from the body wall of the sea cucumber Stichopus japonicus. Carbohydr. Res. 1997, 297, 273–279. [Google Scholar] [CrossRef]
- Luo, L.; Wu, M.; Xu, L.; Lian, W.; Xiang, J.; Lu, F.; Gao, N.; Xiao, C.; Wang, S.; Zhao, J. Comparison of Physicochemical Characteristics and Anticoagulant Activities of Polysaccharides from Three Sea Cucumbers. Mar. Drugs 2013, 11, 399–417. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Xu, L.; Li, J. Preparation and anticoagulant activity of a fucosylated polysaccharide sulfate from a sea cucumber Acaudina molpadioidea. Carbohydr. Polym. 2012, 87, 2052–2057. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Osorio-román, I.O.; Torres, R. Vibrational spectroscopy characterization and anticoagulant activity of a sulfated polysaccharide from sea cucumber Athyonidium chilensis. Carbohydr. Polym. 2012, 88, 959–965. [Google Scholar] [CrossRef]
- Santos, G.R.C.; Glauser, B.F.; Parreiras, L.A.; Vilanova, E. Distinct structures of the α -fucose branches in fucosylated chondroitin sulfates do not affect their anticoagulant activity. Glycobiology 2015, 25, 1–32. [Google Scholar] [CrossRef]
- Ustyuzhanina, A.N.E.; Bilan, M.I.; Dmitrenok, S.; Borodina, E.Y.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. A highly regular fucosylated chondroitin sulfate from the sea cucumber Massinium magnum: Structure and effects on coagulation. Carbohydr. Polym. 2017, 167, 20–26. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Shashkov, A.S.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Structural characterization of fucosylated chondroitin sulfates from sea cucumbers Apostichopus japonicus and Actinopyga mauritiana. Carbohydr. Polym. 2016, 153, 399–405. [Google Scholar] [CrossRef]
- Li, Q.; Cai, C.; Chang, Y.; Zhang, F.; Linhardt, R.J.; Xue, C.; Li, G.; Yu, G. A novel structural fucosylated chondroitin sulfate from Holothuria Mexicana and its effects on growth factors binding and anticoagulation. Carbohydr. Polym. 2017, 181, 1160–1168. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Panina, E.G.; Sanamyan, N.P.; Dmitrenok, A.S.; Tsvetkova, E.A.; Ushakova, N.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure and Anti-Inflammatory Activity of a New Unusual Fucosylated Chondroitin Sulfate from Cucumaria djakonovi. Mar. Drugs 2018, 16, 389. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Nifantiev, N.E.; Usov, A.I. Two fucosylated chondroitin sulfates from the sea cucumber Eupentacta fraudatrix. Carbohydr. Polym. 2017, 164, 8–12. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S. Two structurally similar fucosylated chondroitin sulfates from the holothurian species Stichopus chloronotus and Stichopus horrens. Carbohydr. Polym. 2018, 189, 10–14. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Yang, S.; Lv, Z. Separation, purification, structures and anticoagulant activities of fucosylated chondroitin sulfates from Holothuria scabra. Int. J. Biol. Macromol. 2017, 108, 710–718. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Nifantiev, N.E. Fucosylated chondroitin sulfates from the sea cucumbers Holothuria tubulosa and Holothuria stellati. Carbohydr. Polym. 2018, 200, 1–5. [Google Scholar] [CrossRef]
- Panagos, C.G.; Thomson, D.S.; Moss, C.; Hughes, A.D.; Kelly, M.S.; Liu, Y.; Chai, W.; Venkatasamy, R.; Spina, D.; Page, C.P.; et al. Fucosylated Chondroitin Sulfates from the Body Wall of the Sea Cucumber Holothuria forskali. J. Biol. Chem. 2014, 289, 28284–28298. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. The structure of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria frondosa. Carbohydr. Polym. 2017, 165, 7–12. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Kusaykin, M.I.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Structure and biological activity of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria japonica. Glycobiology 2015, 26, 449–459. [Google Scholar] [CrossRef]
- Boisson-vidal, C.; Tapon-bretaudie, J.; Mourão, P.A.S. Inactivation of Thrombin by a Fucosylated Chondroitin Sulfate from Echinoderm. Thromb. Res. 2001, 102, 167–176. [Google Scholar] [CrossRef]
- Chen, S.; Li, G.; Wu, N.; Guo, X.; Liao, N.; Ye, X.; Liu, D.; Xue, C.; Chai, W. Sulfation pattern of the fucose branch is important for the anticoagulant and antithrombotic activities of fucosylated chondroitin sulfates. BBA Gen. Subj. 2013, 1830, 3054–3066. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Yan, L.; Ding, T.; Linhardt, R.J.; Yu, Y.; Liu, X.; Liu, D.; Ye, X.; Chen, S. Fucosylated chondroitin sulfate oligosaccharides exert anticoagulant activity by targeting at intrinsic tenase complex with low FXII activation: Importance of sulfation pattern and molecular size. Eur. J. Med. Chem. 2017, 139, 191–200. [Google Scholar] [CrossRef]
- Buyue, Y.; Sheehan, J.P. Fucosylated Chondroitin Sulfate Inhibits Plasma Thrombin Generation via Targeting of the Factor IXa Heparin-Binding Exosite. Blood 2009, 114, 3092–3100. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, M.; Xiao, C.; Yang, L.; Zhou, L.; Gao, N.; Li, Z.; Chen, J. Discovery of an intrinsic tenase complex inhibitor: Pure nonasaccharide from fucosylated glycosaminoglycan. Proc. Natl. Acad. Sci. USA 2015, 112, 8284–8289. [Google Scholar] [CrossRef] [Green Version]
- Glauser, B.F.; Pereira, M.S.; Monteiro, R.Q.; Mourão, P.A.S. Serpin-independent anticoagulant activity of a fucosylated chondroitin sulfate. Thromb. Haemost. 2008, 420–428. [Google Scholar] [CrossRef]
- Glauser, B.F.; Mourão, P.A.S.; Pomin, V.H. Marine Sulfated Glycans with Serpin-Unrelated Anticoagulant Properties, 1st ed.; Elsevier Inc.: San Diego, CA, USA, 2013; Volume 62. [Google Scholar]
- Sheehan, J.P.; Walke, E.N. Depolymerized holothurian glycosaminoglycan and heparin inhibit the intrinsic tenase complex by a common antithrombin-independent mechanism. Blood 2019, 107, 3876–3883. [Google Scholar] [CrossRef]
- Pomin, V.H. Anticoagulant motifs of marine sulfated glycans. Glycoconj. J. 2014, 31, 341–344. [Google Scholar] [CrossRef]
- Ben Mansour, M.; Balti, R.; Ollivier, V.; Ben Jannet, H.; Chaubet, F.; Maaroufi, R.M. Characterization and anticoagulant activity of a fucosylated chondroitin sulfate with unusually procoagulant effect from sea cucumber. Carbohydr. Polym. 2017, 174, 760–771. [Google Scholar] [CrossRef]
- Pomin, V.H. Dual and antagonic therapeutic effects of sulfated glycans. Bioorg. Med. Chem. 2016, 24, 3965–3971. [Google Scholar] [CrossRef]
- Minamiguchi, K.; Kitazato, K.T.; Nagase, H.; Sasaki, E.; Ohwada, K.; Kitazato, K. Depolymerized holothurian glycosaminoglycan (DHG), a novel alternative anticoagulant for hemodialysis, is safe and effective in a dog renal failure model. Kidney Int. 2003, 63, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Tovar, A.M.F.; Mourão, P.A.S. High affinity of a fucosylated chondroitin sulfate for plasma low density lipoprotein. Atherosclerosis 1996, 126, 185–195. [Google Scholar] [CrossRef]
- Fonseca, R.J.C.; Mourão, P.A.S. Fucosylated chondroitin sulfate as a new oral antithrombotic agent. Thromb. Haemost. 2006, 96, 822–829. [Google Scholar] [CrossRef]
- Igarashi, M.; Takeda, Y.; Mori, S.; Takahashi, K.; Fuse, T. Depolymerized holothurian glycosaminoglycan (DHG) prevents neointimal formation in balloon-injured rat carotid artery. Atherosclerosis 1997, 129, 27–31. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Ye, X.; Hu, Y.; Ding, T.; Chen, S. Sulfation pattern of fucose branches affects the anti-hyperlipidemic activities of fucosylated chondroitin sulfate. Carbohydr. Polym. 2016, 147, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, D.; Wang, S.; Tao, L.; Wang, A.; Chen, W.; Zhu, Z.; Zheng, S.; Gao, X.; Lu, Y. Holothurian Glycosaminoglycan Inhibits Metastasis and Thrombosis via Targeting of Nuclear Factor- k B/Tissue Factor/Factor Xa Pathway in Melanoma B16F10 Cells. PLoS ONE 2013, 8, e56557. [Google Scholar] [CrossRef]
- He, M.; Wang, J.; Hu, S.; Wang, Y.; Xue, C.; Li, H. The Effects of Fucosylated Chondroitin Sulfate Isolated from Isostichopus badionotus on Antimetastatic Activity via Down- regulation of Hif-1 α and Hpa. Food Sci. Biotechnol. 2014, 23, 1643–1651. [Google Scholar] [CrossRef]
- Pomin, V.H. Antimicrobial Sulfated Glycans: Structure and Function. Curr. Top. Med. Chem. 2017, 17, 319–330. [Google Scholar] [CrossRef]
- Jiang, W.; Li, S.; Hu, S.; Wang, J.; Song, W.; Ji, L.; Wang, Y. Fucosylated Chondroitin Sulfate from Sea Cucumber Inhibited Islets of Langerhans Apoptosis via Inactivation of the Mitochondrial Pathway in Insulin Resistant Mice. Food Sci. Biotechnol. 2015, 24, 1105–1106. [Google Scholar] [CrossRef]
- Monteiro-Machado, M.; Tomaz, M.A.; Fonseca, R.J.C.; Strauch, M.A.; Cons, B.L.; Borges, P.A.; Patr, F.C.; Tavares-Henriques, M.S.; Teixeira-Cruz, J.M.; Calil-Elias, S.; et al. Occurrence of sulfated fucose branches in fucosylated chondroitin sulfate are essential for the polysaccharide effect preventing muscle damage induced by toxins and crude venom from Bothrops jararacussu snake. Toxicon 2015, 98, 20–33. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mathy, M.; Sanchez, C.; Lambert, C. Chondroitin sulfate in the treatment of osteoarthritis: From in vitro studies to clinical recommendations. Ther. Adv. Musculoskelet. Dis. 2010, 2, 335–348. [Google Scholar] [CrossRef]
- Zeng, C.; Wei, J.; Li, H.; Wang, Y.; Xie, D.; Yang, T. Effectiveness and safety of Glucosamine, chondroitin, the two in combination, or celecoxib in the treatment of osteoarthritis of the knee. Sci. Rep. 2015, 5, 16827. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Sang, L.; Wu, D.; Rong, J.; Jiang, L. Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Santos, C.; Sanchez-Pernaute, O.; Largo, R.; Herrero-Beaumont, G. CS/GS Combined Therapy Study Group. Combined Treatment with Chondroitin Sulfate and Glucosamine Sulfate Shows No Superiority Over Placebo for Reduction of Joint Pain and Functional Impairment in Patients with Knee Osteoarthritis. Arthritis Rheumatol. 2017, 69, 77–85. [Google Scholar] [CrossRef]
- Jackson, C.G.; Plaas, A.H.; Sandy, J.D.; Hua, C.; Kim-rolands, S.; Barnhill, J.G.; Harris, C.L.; Clegg, D.O. The human pharmacokinetics of oral ingestion of glucosamine and chondroitin sulfate taken separately or in combination. Osteoarthr. Cartil. 2010, 18, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Volpi, N. Chondroitin Sulfate Safety and Quality. Molecules 2019, 24, 1447. [Google Scholar] [CrossRef]
- Nakano, T.; Ozimek, L. Detection of keratan sulfate by immunological methods in commercial chondroitin sulfate preparations. Carbohydr. Polym. 2014, 99, 547–552. [Google Scholar] [CrossRef]
- Caterson, B.; Melrose, L. Keratan Sulphate, a complex Glycosaminoglycan with Unique Functional Capability. Glycobiology 2018, 28, 182–206. [Google Scholar] [CrossRef]
- Volpi, N. Quality of different chondroitin sulfate preparations in relation to their therapeutic activity. J. Pharm. Pharmacol. 2009, 61, 1271–1280. [Google Scholar] [CrossRef]
- Chevalier, X.; Conrozier, T. Access to highly purified chondroitin sulfate for appropriate treatment of osteoarthritis: A review. Med. Access Point Care 2017, 1. [Google Scholar] [CrossRef]
- Morise, J.; Takematsu, H.; Oka, S. The role of human natural killer-1 (HNK-1) carbohydrate in neuronal plasticity and disease. BBA Gen. Subj. 2017, 1861, 2455–2461. [Google Scholar] [CrossRef]
- Ariga, B.T. Review The role of sulfoglucuronosyl glycosphingolipids in the pathogenesis of monoclonal IgM paraproteinemia and peripheral neuropathy. Proc. Jpn. Acad. Ser. B 2011, 87, 386–404. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Aletras, A.J.; Antonopoulos, C.A.; Hjerpe, A. Determination of the HNK-1 epitope (3-sulphated glucuronic acid) in intact chondroitin sulphates by ELISA. Application to squid skin proteoglycans and their oversulphated carbohydrate structures. Biochimie 1994, 4, 79–82. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Warkentin, T.E. Autoimmune heparin-induced thrombocytopenia. J. Thromb. Haemost. 2017, 15, 2099–2114. [Google Scholar] [CrossRef] [Green Version]
- Blank, M.; Cines, D.B.; Arepally, G.; Eldor, A.; Afek, A. Pathogenicity of human anti-platelet factor 4 (PF4)/heparin in vivo: Generation of mouse anti-PF4/heparin and induction of thrombocytopenia by heparin. Clin. Exp. Immunol. 1997, 108, 333–339. [Google Scholar] [CrossRef]
- Petersen, F.; Brandt, E.; Lindahl, U.; Spillmann, D. Characterization of a Neutrophil Cell Surface Glycosaminoglycan That Mediates Binding of Platelet Factor 4. J. Biol. Chem. 1999, 274, 12376–12382. [Google Scholar] [CrossRef] [Green Version]
- Lord, M.S.; Cheng, B.; Farrugia, B.L.; Mccarthy, S.; Whitelock, J.M. Platelet Factor 4 Binds to Vascular Proteoglycans and Controls Both Growth Factor Activities and Platelet Activation. J. Biol. Chem. 2017, 292, 4054–4063. [Google Scholar] [CrossRef] [Green Version]
- Ramacciotti, E.; Clark, M.; Sadeghi, N.; Hoppensteadt, D.; Thethi, I. Contaminants in Heparin: Review of the Literature, Molecular Profiling, and Clinical Implications. Clin. Appl. Thromb. Hemost. 2011, 17, 126–135. [Google Scholar] [CrossRef]
- Adiguzel, C.; Bansal, V.; Litinas, E.; Cunanan, J.; Iqbal, O.; Nelson, K.; Kannan, M.; Hoppensteadt, D. Increased Prevalence of Antiheparin Platelet Factor 4 Antibodies in Patients May Be Due to Contaminated Heparin. Clin. Appl. Thromb. Hemost. 2008, 15, 145–151. [Google Scholar] [CrossRef]
- Chess, E.K.; Bairstow, S.; Donovan, S.; Havel, K.; Hu, P.; Johnson, R.J.; Lee, S.; Mckee, J.; Miller, R.; Moore, E.; et al. Case Study: Contamination of Heparin with Oversulfated Chondroitin Sulfate. Handb. Exp. Pharmacol. 2012, 207, 99–125. [Google Scholar] [CrossRef]
- Guerrini, M.; Beccati, D.; Shriver, Z.; Naggi, A.; Viswanathan, K.; Bisio, A.; Capila, I.; Lansing, J.C.; Guglieri, S.; Fraser, B.; et al. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat. Biotechnol. 2008, 26, 669–675. [Google Scholar] [CrossRef]
- Kishimoto, T.K.; Viswanathan, K.; Ganguly, T.; Elankumaran, S.; Smith, S.; Pelzer, K. Al-Hakim, A. Contaminated Heparin Associated with Adverse Clinical Events and Activation of the Contact System. N. Engl. J. Med. 2008, 358, 2457–2467. [Google Scholar] [CrossRef]
- Ramacciotti, E.; Wahi, R.; Messmore, H.L. Contaminated Heparin Preparations, Severe Adverse Events and the Contact System. Clin. Appl. Thromb. Hemost. 2008, 14, 489–491. [Google Scholar] [CrossRef]
- Rosania, L. Heparin crisis 2008: A tipping point for increased FDA enforcement in the pharma sector? Food Drug Law J. 2010, 65, 489–501. [Google Scholar]
- Hogwood, J.; Naggi, A.; Torri, G.; Page, C.; Rigsby, P.; Mulloy, B.; Gray, E. The effect of increasing the sulfation level of chondroitin sulfate on anticoagulant specific activity and activation of the kinin system. PLoS ONE 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Van der Meer, J.Y.; Kellenbach, E.; Van den Bos, L.J. From Farm to Pharma: An Overview of Industrial Heparin Manufacturing Methods. Molecules 2017, 22, 1025. [Google Scholar] [CrossRef]
- Pomin, V.H.; Wang, X. Synthetic Oligosaccharide Libraries and Microarray Technology: A Powerful Combination for the Success of Current Glycosaminoglycan Interactomics. ChemMedChem 2017, 13, 648–661. [Google Scholar] [CrossRef] [Green Version]
- Cella, G.; Boeri, G.; Saggiorato, G.; Paolini, R.; Luzzatto, G.; Terribile, V.I. Interaction between Histidine-Rich Glycoprotein and Platelet Factor 4 with Dermatan Sulfate and Low-Molecular-Weight Dermatan Sulfate. Angiology 1992, 43, 59–62. [Google Scholar] [CrossRef]
- Vitale, C.; Berutti, S.; Bagnis, C. Dermatan sulfate: An alternative to unfractionated heparin for anticoagulation in hemodialysis patients. J. Nephrol. 2013, 26, 158–163. [Google Scholar] [CrossRef]
- Greinacher, M.D.A. Clinical Practice. Heparin-Induced Thrombocytopenia. N. Engl. J. Med. 2015, 373, 252–261. [Google Scholar] [CrossRef]
- Pan, J.; Qian, Y.; Zhou, X.; Lu, H.; Ramacciotti, E.; Zhang, L. Chemically Oversulfated Glycosaminoglycans Are Potent Modulators of Contact System Activation and Different Cell Signaling Pathways. J. Biol. Chem. 2010, 285, 22966–22975. [Google Scholar] [CrossRef] [Green Version]
- Sommers, C.D.; Montpas, N.; Adam, A.; Keire, D.A. Characterization of currently marketed heparin products: Adverse event relevant bioassays. J. Pharm. Biomed. Anal. 2012, 67, 28–35. [Google Scholar] [CrossRef]
- Fonseca, R.J.C.; Oliveira, S.M.C.G.; Pomin, V.H.; Araujo, I.G.; Mourão, P.A. Effects of oversulfated and fucosylated chondroitin sulfates on coagulation Challenges for the study of anticoagulant polysaccharides. Thromb. Haemost. 2010, 103, 994–1004. [Google Scholar] [CrossRef]
- Lin, L.; Xu, L.; Xiao, C.; Zhou, L.; Gao, N.; Wu, M.; Zhao, J. Plasma contact activation by a fucosylated chondroitin sulfate and its structure-activity-relationship study. Glycobiology 2018, 28, 754–764. [Google Scholar] [CrossRef]
- Pang, M.; Siddiquee, S.; Al Azad, S. Fucosylated chondroitin sulfate diversity in sea cucumbers: A review. Carbohydr. Polym. 2014, 112, 173–178. [Google Scholar] [CrossRef]






| Type | Structure | Source | Reference |
|---|---|---|---|
| CS-A | GlcA(β1−3)GalNAc(4OSO3−) | Whale | [58] |
| CS-C | GlcA(β1−3)GalNAc(6OSO3−) | Shark | [59] |
| CS-D | GlcA(2OSO3−)(β1−3)GalNAc(6OSO3−) | Fish, Shark, Squid | [47,52] |
| CS-E | GlcA(β1−3)GalNAc(4,6diOSO3−) | Squid | [46,60] |
| CS-K | GlcA(3OSO3−)(β1−3)GalNAc(4OSO3−) | King Crab and Octopus | [46,61,62] |
| CS-L | GlcA(3OSO3−)(β1−3)GalNAc(6OSO3−) | Squid | [46] |
| CS-M | GlcA(3OSO3−)(β1−3)GalNAc(4,6diOSO3−) | Squid | [46] |
| Type | Structure | Source | Reference |
|---|---|---|---|
| DS (4S) | IdoA(α1−3)GalNAc(4OSO3−) | Bovine and porcine mucosa | [108] |
| DS (2S4S) | IdoA(2OSO3−)(α1−3)GalNAc(4OSO3−) | Tunic of ascidian | [104,109] |
| DS (6S) | IdoA(α1−3)GalNAc(6OSO3−) | Bovine liver, bovine spleen, rabbit liver and hog spleen | [58] |
| DS (2S6S) | IdoA(2OSO3−)(α1−3)GalNAc(6OSO3−) | Tunic of ascidian | [105] |
| DS (2S4,6S) (CS-H) | IdoA(2OSO3−)(α1−3)GalNAc(4,6diOSO3−) | Hagfish skin and Hagfish notochord | [72,110,111,112] |
| Species | Fuc0S | Fuc3S | Fuc4S | Fuc2S4S | Fuc3S4S | References |
|---|---|---|---|---|---|---|
| Ludwigothurea griseaa | 0 | − | ~49 | ~20 | ~17 | [19,165] |
| Pearsonothuria graeffei | − | − | 81.6 | 18.4 | − | [166] |
| Holothuriava gabunda | 25.6 | − | 50.2 | 15.8 | 8.4 | [166] |
| Stichopus tremulus | − | − | 24.8 | 22.4 | 52.8 | [166] |
| Isostichopus badionotus | − | − | 4.1 | 95.9 | − | [166] |
| Thelenota ananas | 0 | ~25 | ~22 | ~53 | 0 | [167,168] |
| Stichopus japonicusb | 18 | 17 c | 0 | 16 | 23 | [169] |
| Holothuria edulisd | − | − | Nd | 18 | Nd | [170] |
| Apostichopus japonicusd | − | − | Nd | 45 | Nd | [170] |
| Holothuria nobilise | − | Nd | Nd | − | Nd | [170] |
| Acaudina molpadioideaf | − | − | − | − | − | [171] |
| Athyonidium chilensisf | − | − | − | − | − | [172] |
| Patalus mollis | 0 | 0 | 26 | 34 | 40 | [173] |
| Massinium magnumg | _ | _ | _ | _ | Nd | [174] |
| Apostichopus mauritania | _ | _ | _ | 16.7 | 66.7 | [175] |
| Holothuria mexicana | 6.24 | 5.58 | 37.16 | _ | 51 | [176] |
| Cucumaria djakonovi | _ | _ | ~25 | ~50 | ~25 | [177] |
| Eupentaca fraudatrixh | 33.3 | _ | _ | _ | 66.7 | [178] |
| Stichopus horrensi | _ | _ | _ | Nd | _ | [179] |
| Stichopus chlorontusi | _ | _ | _ | Nd | _ | [179] |
| Holothuria scabraj | _ | _ | 27.1 | 72.9 | _ | [180] |
| Holothuria tubulosak | _ | _ | 14.3 | 42.85 | 42.85 | [181] |
| Holothuria stellatik | _ | _ | 20 | 40 | 40 | [181] |
| Holothuria forskai | _ | _ | 15 | 39 | 46 | [182] |
| Cucumaria frondosal | _ | _ | _ | 25 | 62.5 | [183] |
| Cucumaria japonica | _ | _ | _ | 20 | 80 | [184] |
| Actinopyga mauritiana | _ | _ | _ | 20 | 80 | [175] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomin, V.H.; Vignovich, W.P.; Gonzales, A.V.; Vasconcelos, A.A.; Mulloy, B. Galactosaminoglycans: Medical Applications and Drawbacks. Molecules 2019, 24, 2803. https://doi.org/10.3390/molecules24152803
Pomin VH, Vignovich WP, Gonzales AV, Vasconcelos AA, Mulloy B. Galactosaminoglycans: Medical Applications and Drawbacks. Molecules. 2019; 24(15):2803. https://doi.org/10.3390/molecules24152803
Chicago/Turabian StylePomin, Vitor H., William P. Vignovich, Alysia V. Gonzales, Ariana A. Vasconcelos, and Barbara Mulloy. 2019. "Galactosaminoglycans: Medical Applications and Drawbacks" Molecules 24, no. 15: 2803. https://doi.org/10.3390/molecules24152803
APA StylePomin, V. H., Vignovich, W. P., Gonzales, A. V., Vasconcelos, A. A., & Mulloy, B. (2019). Galactosaminoglycans: Medical Applications and Drawbacks. Molecules, 24(15), 2803. https://doi.org/10.3390/molecules24152803