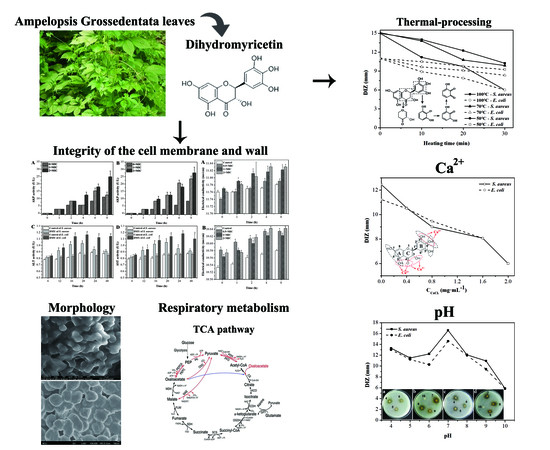
Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of Dihydromyricetin (DMY)
2.2. Effect of Calcium Ions, Thermal Processing, and pH on the Antibacterial Activity of DMY
2.3. Morphology of Bacteria Treated with DMY
2.4. Integrity of Cell Membranes and Walls
2.5. Permeability of Cell Membrane
2.6. Oxidative Respiratory Metabolism Characteristics
3. Materials and Methods
3.1. Plant Material
3.2. Microorganisms
3.3. Extraction, Purification, and Determination of DMY
3.4. Antibacterial Activity
3.5. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.6. Scanning Electron Microscopy (SEM) Observations
3.7. Determination of Extracellular Enzyme Activity
3.8. Determination of the Bacterial Fluid Conductivity
3.9. Determination of Oxidative Respiratory Metabolism
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS. Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2018, 99, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.M.; Lee, J.; Chen, W.N. Potential natural food preservatives and their sustainable production in yeast: Terpenoids and polyphenols. J. Agric. Food Chem. 2019, 67, 4397–4417. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An overview of their antimicrobial activity against human infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E-coli O157: H7, Salmonella Typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Cegielska-Radziejewska, R.; Lesnierowski, G.; Kijowski, J. Antibacterial activity of hen egg white lysozyme modified by thermochemical technique. Eur. Food Res. Technol. 2009, 228, 841–845. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Muhammad, Y.; Imran, K.; Bahadur, K.S.; Naveed, A.; Hussain, J.E. Lactoperoxidase immobilization on silver nanoparticles enhances its antimicrobial activity. J. Dairy Res. 2018, 85, 460–464. [Google Scholar]
- Oyinloye, B.; Adenowo, A.; Kappo, A. Reactive oxygen species, apoptosis, antimicrobial peptides and human inflammatory diseases. Pharmaceuticals 2015, 8, 151–175. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Zhang, H.B.; Xie, G.Y.; Tian, M.; Pu, Q.; Qin, M.J. Optimization of the ultrasonic-assisted extraction of bioactive flavonoids from Ampelopsis Grossedentata and subsequent separation and purification of two flavonoid aglycones by high-speed counter-current chromatography. Molecules 2016, 21, 1960. [Google Scholar] [CrossRef]
- Tang, N.N.; Ma, J.; Wang, K.S.; Mi, C.L.; Lv, Y.; Piao, L.X.; Jin, X.J. Dihydromyricetin suppresses TNF-alpha-induced NF-kappa B activation and target gene expression. Mol. Cell Biochem. 2016, 422, 11–20. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Zhang, H.Q.; Chen, S.Y.; Xu, Y.; Yao, A.J.; Liao, Q.; Zhang, X.H. Dihydromyricetin induces mitochondria-mediated apoptosis in HepG2 cells through down-regulation of the Akt/Bad pathway. Nutr. Res. 2017, 38, 27–33. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, A.R.; Liu, B.G.; Li, G.R.; Zhang, Z.P.; Chen, S.J. Antioxidant and cytotoxic activity of dihydromyricetin from Ampelopsis Grossedentata leaves. Agro Food Ind. Hi-Tech 2009, 20, 14–17. [Google Scholar]
- Ren, Z.X.; Zhao, Y.F.; Cao, T.; Zhen, X.C. Dihydromyricetin protects neurons in an MPTP-induced model of Parkinson’s disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacol. Sin. 2017, 38, 733. [Google Scholar] [CrossRef]
- Jarrod, W.; Charles, E.; Scott, G.; Rebecca, S.; Robert, L. Bsn723t prevents atherosclerosis and weight gain in apoE knockout mice fed a western diet. Webmedcentral 2015, 6, 5034. [Google Scholar]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Gao, H. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2016, 218, 463–470. [Google Scholar] [CrossRef]
- Gosch, C.; Nagesh, K.M.; Thill, J.; Miosic, S.; Plaschil, S.; Milosevic, M. Isolation of dihydroflavonol 4-reductase cdna clones from angelonia x angustifolia and heterologous expression as gst fusion protein in Escherichia coli. PLoS ONE 2014, 9, e107755. [Google Scholar] [CrossRef]
- Xing, H.P.; Ji, H.W.; Yang, W.L.; Zhang, Y.S. Antimicrobial activity of extracts from Ampelopsis Grossedentata on common respiratory tract pathogens. J. Guangxi Agric. Biol. Sci. 2007, 26, 150–153. [Google Scholar]
- Ameen, F.; Alyahya, S.A.; Bakhrebah, M.A.; Nassar, M.S.; Aljuraifani, A. Flavonoid dihydromyricetin-mediated silver nanoparticles as potential nanomedicine for biomedical treatment of infections caused by opportunistic fungal pathogens. Res. Chem. Intermed. 2018, 44, 1–11. [Google Scholar] [CrossRef]
- Dalcin, A.J.F.; Santos, C.G.; Gündel, S.S.; Roggia, I.; Raffin, R.P.; Ourique, A.F.; Santos, R.C.V.; Gomes, P. Anti biofilm effect of dihydromyricetin-loaded nanocapsules on urinary catheter infected by Pseudomonas aeruginosa. Colloid Surf. B 2017, 156, 282–291. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S.; Sun, P.L.; Luo, S.L. Antibacterial activity and mechanisms of essential oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef]
- Echeverria, J.; Opazo, J.; Mendoza, L.; Urzua, A.; Wilkens, M. Structure-activity and lipophilicity relationships of selected antibacterial natural flavones and flavanones of Chilean flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef]
- Woznicka, E.; Kuzniar, A.; Nowak, D.; Nykiel, E.; Kopacz, M.; Gruszecka, J.; Golec, K. Comparative study on the antibacterial activity of some flavonoids and their sulfonic derivatives. Acta Pol. Pharm. 2013, 70, 567–571. [Google Scholar]
- Wu, Y.P.; Bai, J.R.; Zhong, K.; Bai, D.D.; Huang, Y.N.; Xiao, K.; Gao, H. Antibacterial effect of 2R,3R-dihydromyricetin on the cellular functions of Staphylococcus aureus. Biosci. Biotech. Bioch. 2018, 82, 135–138. [Google Scholar] [CrossRef]
- Shakeri, A.; Khakdan, F.; Soheili, V.; Sahebkar, A.; Rassam, G.; Asili, J. Chemical composition, antibacterial activity, and cytotoxicity of essential oil from Nepeta ucrainica L. spp. kopetdaghensis. Ind. Crops Prod. 2014, 58, 315–321. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Liu, C.S.; Liu, C.G.; Meng, X.H.; Yu, L.J. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloid Surf. B 2008, 65, 197–202. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Carreon-Alvarez, A.; Barcena-Soto, M.; Casillas, N. Metals in alcoholic beverages: A review of sources, effects, concentrations, removal, speciation, and analysis. J. Food Compos. Anal. 2008, 21, 672–683. [Google Scholar] [CrossRef]
- Ying, L.S.; Hua, G.J.; Quan, G.Q.; Xiang, N.Z. Stability of dihydromyricetin and factors affecting the stability. J. Wuxi Univ. Light Ind. 2004, 23, 17–20. [Google Scholar]
- Malesev, D.; Kuntic, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Sun, D.W.; Wang, L. Recent developments in numerical modelling of heating and cooling processes in the food industry: A review. Trends Food Sci. Tech. 2003, 14, 408–423. [Google Scholar]
- Xin, M.; Ma, Y.; Lin, W.; Xu, K.; Chen, M. Study on the structure–activity of dihydromyricetin and its new production. J. Therm. Anal. Calorim. 2014, 116, 241–248. [Google Scholar] [CrossRef]
- Fan, L.L.; He, L.L.; Wei, W.; Cao, F.; Zhang, Y.Z.; Miao, J.H. Content determination of dihydromyricetin and myricetin in leaves of Ampelopsis Grossedentata by UPLC and investigation of their thermal stability. China Pharm. 2012, 23, 3316–3319. [Google Scholar]
- Zhang, P.P.; Cai, S.N.; Song, L.; Zhang, L.Q.; Fan, H.H.; Zhou, L.; Lin, R.; Yang, G.; Bian, X.L.; Wang, W.R.; et al. Solubility of dihydromyricetin in ethanol and water mixtures from 288.15 to 323.15k. J. Mol. Liq. 2015, 211, 197–202. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F. Bacterial membrane lipids in the action of antimicrobial agents. J. Pept. Sci. 2011, 17, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Wang, C.G.; Wang, W.Q.; Shi, C.Y.; Fang, J.G. Gastrointestinal stability of dihydromyricetin, myricetin, and myricitrin: An in vitro investigation. Int. J. Food Sci. Nutr. 2017, 68, 1–11. [Google Scholar] [CrossRef]
- Li, K.C.; Xing, R.G.; Liu, S.; Qin, Y.K.; Yu, H.H.; Li, P.C. Size and pH effects of chitooligomers on antibacterial activity against Staphylococcus aureus. Int. J. Biol. Macromol. 2014, 64, 302–305. [Google Scholar] [CrossRef]
- El-Maati, M.F.A.; Mahgoub, S.A.; Labib, S.M.; Al-Gaby, A.M.A.; Ramadan, M.F. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur. J. Integr. Med. 2016, 8, 494–504. [Google Scholar] [CrossRef]
- Anwar, A.; Masri, A.; Rao, K.; Rajendran, K.; Khan, N.A.; Shah, M.R.; Siddiqui, R. Antimicrobial activities of green synthesized gums-stabilized nanoparticles loaded with flavonoids. Sci. Rep. 2019, 9, 3122. [Google Scholar] [CrossRef]
- Tang, H.; Chen, W.; Dou, Z.M.; Chen, R.; Hu, Y.; Chen, W.; Chen, H. Antimicrobial effect of black pepper petroleum ether extract for the morphology of Listeria monocytogenes and Salmonella typhimurium. J. Food Sci. Technol. 2017, 54, 2067–2076. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, S.; Wu, W. Preliminary studies on the antibacterial mechanism of yanglingmycin. Pestic. Biochem. Phys. 2017, 147, 27–31. [Google Scholar] [CrossRef]
- Diao, M.M.; Qi, D.P.; Xu, M.M.; Lu, Z.X.; Lv, F.X.; Bie, X.M.; Zhang, C.; Zhao, H.Z. Antibacterial activity and mechanism of monolauroyl-galactosylglycerol against Bacillus cereus. Food Control 2018, 85, 339–344. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, K.P.; Zhang, X.; Pan, D.D.; Sun, Y.Y.; Cao, J.X. Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli. Front. Microbiol. 2017, 7, 2094. [Google Scholar] [CrossRef]
- Wang, Z.C.; Chen, P.Z.; Tao, N.; Zhang, H.R.; Li, R.F.; Zhan, X.B.; Wang, F.Z.; Shen, Y.B. Anticancer activity of polysaccharides produced from glycerol and crude glycerol by an endophytic fungus Chaetomium globosum CGMCC 6882 on human lung cancer A549 cells. Biomolecules 2018, 8, 171. [Google Scholar] [CrossRef]
- Zhu, H.L.; Chen, G.; Chen, S.N.; Wang, Q.R.; Wan, L.; Jian, S.P. Characterization of polyphenolic constituents from Sanguisorba officinalis L. and its antibacterial activity. Eur. Food Res. Technol. 2019, 245, 1487–1498. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Song, L.; Li, T.; Jia, Y. Antimicrobial activity and mechanism of larch bark procyanidins against Staphylococcus aureus. Acta Bioch. Bioph. Sin. 2017, 49, 1–9. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.L.; Li, C.Z.; Cui, H.Y. Antibacterial mechanism of ε-Poly-lysine against Listeria monocytogenes and its application on cheese. Food Control 2018, 91, 76–84. [Google Scholar] [CrossRef]
- Clara, R.; Langhans, W.; Mansouri, A. Oleic acid stimulates glucagon-like peptide-1 release from enteroendocrine cells by modulating cell respiration and glycolysis. Metabolism 2016, 65, 8–17. [Google Scholar] [CrossRef]
- Cui, H.Y.; Zhang, C.H.; Li, C.Z.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Gao, J.; Liu, B.; Ning, Z.; Zhao, R.; Zhang, A.; Qiong, W.U. Characterization and antioxidant activity of flavonoid-rich extracts from leaves of Ampelopsis Grossedentata. J. Food Biochem. 2009, 33, 808–820. [Google Scholar] [CrossRef]
- Liao, W.Z.; Ning, Z.X.; Ma, L.; Yin, X.R.; Wei, Q.Y.; Yuan, E.D.; Ren, J.Y. Recrystallization of dihydromyricetin from Ampelopsis Grossedentata and its anti-oxidant activity evaluation. Rejuv. Res. 2014, 17, 422–429. [Google Scholar] [CrossRef]
- He, G.X.; Pei, G.; Yang, W.L.; Li, B. Determination of dihydromyricetin in different parts of Ampelopsis Grossedentata in different seasons by HPLC. Chin. Tradit. Pat. Med. 2004, 26, 210–212. [Google Scholar]
- Arima, H.; Ashida, H.; Danno, G. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotech. Bioch. 2002, 66, 1009–1014. [Google Scholar] [CrossRef]
- Duffy, L.L.; Osmond-McLeod, M.J.; Judy, J.; King, T. Investigation into the antibacterial activity of silver, zinc oxide and copper oxide nanoparticles against poultry-relevant isolates of Salmonella and Campylobacter. Food Control 2018, 92, 293–300. [Google Scholar] [CrossRef]
- Vaquero, M.J.R.; Serravalle, L.R.T.; De-Nadra, M.C.M.; De-Saad, A.M.S. Antioxidant capacity and antibacterial activity of phenolic compounds from argentinean herbs infusions. Food Control 2010, 21, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.Y.; Zhang, X.J.; Zhou, H.; Zhao, C.T.; Lin, L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef]
- Crowley, L.V. The Reitman-Frankel colorimetric transaminase procedure in suspected myocardial infarction. Clin. Chem. 1967, 13, 482–487. [Google Scholar]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.Z.; Dai, J.M.; Cui, H.Y.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
Sample Availability: Samples of the dihydromyricetin are available from the authors. |






| Bacteria | DIZ (mm) | DMY (mg/mL) | BH (mg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 3.78 mg/mL | 7.56 mg/mL | 11.34 mg/mL | MIC | MBC | MIC | MBC | |
| Gram positive | ||||||||
| S. aureus | ND | 10.6 ± 0.5 | 15 ± 0.2 | 18 ± 0.3 | 0.625 | 2.5 | 0.625 | 2.5 |
| B. subtilis | ND | 8.1 ± 0.2 | 10.3 ± 0.4 | 12.3 ± 0.3 | 1.25 | 10 | 1.25 | 10 |
| Gram negative | ||||||||
| E. coli | ND | 9.8 ± 0.3 | 12.8 ± 0.3 | 15.5 ± 0.6 | 0.3125 | 2.5 | 0.625 | 5 |
| S. paratyphi | ND | 10.5 ± 0.3 | 11.2 ± 0.4 | 13.1 ± 0.5 | 0.625 | 2.5 | 1.25 | 5 |
| P. aeruginosa | ND | 10.1 ± 0.3 | 13.2 ± 0.3 | 16.3 ± 0.4 | 0.3125 | 2.5 | 0.3125 | 2.5 |
| Inhibitors | S. aureus | E. coli | ||
|---|---|---|---|---|
| IR/% | SR/% | IR/% | SR/% | |
| DMY | 20.62 | 19.24 | ||
| Malonic acid | 28.26 | 11.47 | 24.18 | 9.62 |
| Iodoacetic acid | 7.47 | 17.16 | 8.26 | 15.47 |
| Sodium phosphate tribasic dodecahydrate | 19.18 | 24.96 | 17.26 | 19.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.-N.; Wang, F.; Yuan, Y.-T.; Liu, J.; Liu, Y.-Z.; Yi, X. Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria. Molecules 2019, 24, 2831. https://doi.org/10.3390/molecules24152831
Xiao X-N, Wang F, Yuan Y-T, Liu J, Liu Y-Z, Yi X. Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria. Molecules. 2019; 24(15):2831. https://doi.org/10.3390/molecules24152831
Chicago/Turabian StyleXiao, Xiao-Nian, Fan Wang, Yi-Ting Yuan, Jing Liu, Yue-Zhen Liu, and Xing Yi. 2019. "Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria" Molecules 24, no. 15: 2831. https://doi.org/10.3390/molecules24152831
APA StyleXiao, X. -N., Wang, F., Yuan, Y. -T., Liu, J., Liu, Y. -Z., & Yi, X. (2019). Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria. Molecules, 24(15), 2831. https://doi.org/10.3390/molecules24152831