Chemical Composition, Antioxidant and Cytotoxicity Activities of Leaves, Bark, Twigs and Oleo-Resin of Dipterocarpus alatus
Abstract
:1. Introduction
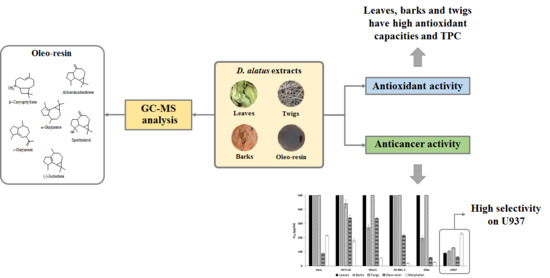
2. Results and Discussion
2.1. Extraction and Phytochemical Analysis
2.2. Chemical Constituents of D. alatus Extracts
2.3. Cytotoxic Activity
2.4. Antioxidant Activities and Total Phenolic Contents
3. Materials and Methods
3.1. Plant Materials
3.2. Sample Preparation
3.3. Phytochemical Test
3.4. Antioxidant Activity
3.4.1. DPPH Radical Scavenging
3.4.2. ABTS Radical Scavenging
3.4.3. Ferric Reducing Antioxidant Power (FRAP)
3.4.4. Total Phenolic Content (TPC)
3.5. Cell Culture
3.6. Cytotoxic Activity
3.7. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.8. Statistical Analysis
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Virani, S.; Bilheem, S.; Chansaard, W.; Chitapanarux, I.; Daoprasert, K.; Khuanchana, S.; Leklob, A.; Pongnikorn, D.; Rozek, L.S.; Siriarechakul, S.; et al. National and subnational population-based incidence of cancer in Thailand: Assessing Cancers with the Highest Burdens. Cancers 2017, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- de Silva, M.B.; Tencomnao, T. The protective effect of some Thai plants and their bioactive compounds in UV light-induced skin carcinogenesis. J. Photochem. Photobiol. B 2018, 185, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Mussarat, S.; Adnan, M. Review on ethnomedicinal, phytochemical and pharmacological evidence of Himalayan anticancer plants. J. Ethnopharmacol. 2015, 164, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.D.; Zou, Y.P.; Wang, P.; Yao, X.H.; Sun, Y.; Duan, M.H.; Fu, Y.J.; Yu, B. Chimaphilin induces apoptosis in human breast cancer MCF-7 cells through a ROS-mediated mitochondrial pathway. Food Chem. Toxicol. 2014, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Zuraida, W.; Zain, W.M.; Ahmat, N.; Norizan, N.H.; Ainaa, N.; Nazri, A.M. The evaluation of antioxidant, antibacterial and structural identification activity of trimer resveratrol from Malaysia’s Dipterocarpaceae. Aust. J. Basic Appl. Sci. 2011, 5, 926–929. [Google Scholar]
- Khiev, P.; Kwon, O.K.; Song, H.H.; Oh, S.R.; Ahn, K.S.; Lee, H.K.; Chin, Y.W. Cytotoxic terpenes from the stems of Dipterocarpus obtusifolius collected in Cambodia. Chem. Pharm. Bull. 2012, 60, 955–961. [Google Scholar] [CrossRef]
- Yang, W.S.; Lee, B.H.; Kim, S.H.; Kim, H.G.; Yi, Y.S.; Htwe, K.M.; Kim, Y.D.; Yoon, K.D.; Hong, S.; Lee, W.S.; et al. Dipterocarpus tuberculatus ethanol extract strongly suppresses in vitro macrophage-mediated inflammatory responses and in vivo acute gastritis. J. Ethnopharmacol. 2013, 146, 873–880. [Google Scholar] [CrossRef]
- Senathilake, K.S.; Karunanayake, E.H.; Samarakoon, S.R.; Tennekoon, K.H.; de Silva, E.D.; Adhikari, A. Oleanolic acid from antifilarial triterpene saponins of Dipterocarpus zeylanicus induces oxidative stress and apoptosis in filarial parasite Setaria digitata in vitro. Exp. Parasitol. 2017, 177, 13–21. [Google Scholar] [CrossRef]
- Akter, R.; Uddin, S.J.; Grice, I.D.; Tiralongo, E. Cytotoxic activity screening of Bangladeshi medicinal plant extracts. J. Nat. Med. 2014, 68, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tanaka, T.; Iinuma, M.; Nakaya, K.; Takahashi, Y.; Sawa, R.; Murata, J.; Darnaed, D. Two new resveratrol (=5-[(1E)-2-(4-Hydroxyphenyl)ethenyl]benzene-1,3-diol) tetramers with a tetrahydrofuran ring from Dipterocarpus grandiflorus. Helv. Chim. Acta 2004, 87, 479–495. [Google Scholar] [CrossRef]
- Bisset, N.G.; Diaz, M.A.; Ehret, C.; Ourisson, G.; Palmade, M.; Patil, F.; Pesnelle, P.; Streith, J. Etudes chimio-taxonomiques dans la famille des diptérocarpacées-II.: Constituants du genre Diptérocarpus gaertn. F. Essai de classification chimio-taxonomique. Phytochemistry 1966, 5, 865–880. [Google Scholar] [CrossRef]
- Muhtadi; Hakim, E.H.; Juliawaty, L.D.; Syah, Y.M.; Achmad, S.A.; Latip, J.; Ghisalberti, E.L. Cytotoxic resveratrol oligomers from the tree bark of Dipterocarpus hasseltii. Fitoterapia 2006, 77, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Chen, C.J.; Yan, W.; Ge, H.M.; Kong, L.D. Anti-hyperuricemic and anti-inflammatory actions of vaticaffinol isolated from Dipterocarpus alatus in hyperuricemic mice. Chin. J. Nat. Med. 2017, 15, 330–340. [Google Scholar] [CrossRef]
- Chen, C.J.; Jiang, R.; Wang, G.; Jiao, R.H.; Tancharoen, C.; Sudto, K.; Vajarothai, S.; Hannongbua, S.; Ge, H.M.; Tan, R.X. Oligostilbenoids with acetylcholinesterase inhibitory activity from Dipterocarpus alatus. Planta Med. 2014, 80, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Appanah, S.; Turnbull, J.M. A Review of Dipterocarps: Taxonomy, Ecology and Silviculture; Center for international forestry research: Bogor, Indonesia, 1998; pp. 189–191. [Google Scholar]
- Aslam, M.S.; Ahmad, M.S.; Mamat, A.S. A phytochemical, ethnomedicinal and pharmacological review of genus Dipterocarpus. Int. J. Pharm. Pharm. Sci. 2015, 7, 27–38. [Google Scholar]
- Wiart, C. Medicinal Plants of the Asia-Pacific: Drugs for the Future? World scientific publishing Co. Pte. Ltd.: Singapore, 2006; pp. 139–140. [Google Scholar]
- Prasad, P.R.C. Ecological analysis of Dipterocarpaceae of north andaman forest. India J. Plant. Develop. 2011, 18, 135–149. [Google Scholar]
- Karnick, C.R.; Hocking, G.M. Ethnobotanical records of drug plants described in valmiki ramayana and their uses in the ayurvedic system of medicine. Quart. J. Crude Drug Res. 1974, 13, 143–154. [Google Scholar] [CrossRef]
- Jantan, I.B. The essential oil of Dipterocarpus kerrii. J. Trop. For. Sci. 1988, 1, 11–15. [Google Scholar]
- Messer, A.; McCormick, K.; Hagedorn, S.H.H.; Tumbel, F.; Meinwald, J. Defensive role of tropical tree resins: Antitermitic sesquiterpenes from Southeast Asian Dipterocarpaceae. J. Chem. Ecol. 1990, 16, 3333–3352. [Google Scholar] [CrossRef] [PubMed]
- Bézivin, C.; Tomasi, S.; Lohézic-Le Dévéhat, F.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Shafaei, A.; Jafari, S.F.; Mohamed, S.K.; Ezzat, M.O.; Abdul Majid, A.S.; Oon, C.E.; Petersen, S.H.; Kono, K.; Abdul Majid, A.M. Isoledene from Mesua ferrea oleo-gum resin induces apoptosis in HCT 116 cells through ROS-mediated modulation of multiple proteins in the apoptotic pathways: A mechanistic study. Toxicol. Lett. 2016, 257, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.S.; Youssef, D.T.A.; Sylvester, P.W.; Wali, V.; El Sayed, K.A. Antiproliferative Sesquiterpenes from the Red Sea Soft Coral Sarcophyton glaucum. Nat. Prod. Commun. 2007, 2, 1–3. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β- Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Manosroi, J.; Boonpisuttinant, K.; Manosroi, W.; Manosroi, A. Anti-proliferative activities on HeLa cancer cell line of Thai medicinal plant recipes selected from MANOSROI II database. J. Ethnopharmacol. 2012, 142, 422–431. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.; Juraimi, A.; Tayebi-Meigooni, A. Comparative evaluation of different extraction techniques and solvents for the assay of phytochemicals and antioxidant activity of Hashemi Rice Bran. Molecules 2015, 20, 10822–10838. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods. 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Srisayam, M.; Weerapreeyakul, N.; Barusrux, S.; Tanthanuch, W.; Thumanu, K. Application of FTIR microspectroscopy for characterization of biomolecular changes in human melanoma cells treated by sesamol and kojic acid. J. Dermatol. Sci. 2014, 73, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Gelmini, F.; Beretta, G.; Anselmi, C.; Centini, M.; Magni, P.; Ruscica, M.; Cavalchini, A.; Maffei Facino, R. GC-MS profiling of the phytochemical constituents of the oleoresin from Copaifera langsdorffii Desf. and a preliminary in vivo evaluation of its antipsoriatic effect. Int. J. Pharm. 2013, 440, 170–178. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Dipterocarpus alatus extracts are available from the authors. |

| Part of Plant | Chemical Group | ||||
|---|---|---|---|---|---|
| Alkaloid | Steroid | Tannin | Xanthone | Reducing Sugar | |
| Leaves | − | − | + | − | + |
| Bark | + | + | + | + | + |
| Twigs | − | + | + | − | + |
| Oleo-resin | − | + | − | − | − |
| Compound | Retention Time | Compound Name | Molecular Weight | Molecular Formula | % (Peak area) | Fragmentation Pattern m/z (Decreasing Order of Abundance) |
|---|---|---|---|---|---|---|
| 1 | 23.48 | δ-Elemene | 204 | C15H24 | 0.08 | 121(100), 93, 136, 107, 79, 41, 161, 55, 67, 189, 204 |
| 2 | 24.05 | α-Cubebene | 204 | C15H24 | 0.03 | 105(100), 119, 161, 91, 81, 41, 55, 204, 133 |
| 3 | 25.25 | α-Copeane | 204 | C15H24 | 0.35 | 119(100), 105, 161, 93, 81, 41, 55, 133, 204, 65 |
| 4 | 25.98 | β-Elemene | 204 | C15H24 | 0.78 | 93(100), 81, 68, 107, 41, 55, 121, 147, 133, 161, 189, 175, 204 |
| 5 | 26.87 | α-Gurjunene | 204 | C15H24 | 30.31 | 105(100), 119, 161, 204, 189, 91, 133, 41, 147, 81, 55, 67 |
| 6 | 27.10 | β-Caryophyllene | 204 | C15H24 | 3.14 | 93(100), 69, 41, 133, 105, 79, 55, 119, 147, 161, 189, 175, 204 |
| 7 | 27.63 | (−)-Isoledene | 204 | C15H24 | 13.69 | 161(100), 105, 119, 91, 41, 133, 189, 147, 69, 69, 55, 204 |
| 8 | 28.26 | α-Humulene | 204 | C15H24 | 0.94 | 93(100), 80, 121, 41, 147, 107, 67, 55, 204, 136 |
| 9 | 28.55 | Alloaromadendrene | 204 | C15H24 | 3.28 | 91(100), 41, 105, 161, 133, 79, 119, 65, 55, 147, 204, 189 |
| 10 | 28.94 | γ-Gurjunene | 204 | C15H24 | 3.14 | 107(100), 81, 161, 91, 121, 41, 55, 133, 67, 189, 147, 204, 175 |
| 11 | 29.65 | Viridiflorene | 204 | C15H24 | 0.61 | 107(100), 93, 41, 119, 81, 133, 55, 161, 189, 67, 147, 204, 175 |
| 12 | 29.96 | β-Vatirenene | 202 | C15H22 | 0.12 | 145(100), 105, 131, 91, 202, 120, 159, 187, 41, 77, 55, 67, 173 |
| 13 | 30.37 | Selina-3,7(11)-diene | 204 | C15H24 | 0.52 | 122(100), 161, 107, 91, 81, 41, 55, 67, 133, 204, 189, 147 |
| 14 | 31.65 | Calarene epoxide | 220 | C15H24O | 0.04 | 41(100), 65, 93, 109, 119, 82, 135, 161, 185, 145, 177, 205, 220 |
| 15 | 31.85 | Palustrol | 222 | C15H26O | 0.13 | 122(100), 111, 81, 93, 41, 67, 161, 147, 189, 204, 133, 175, 222 |
| 16 | 32.17 | Spathulenol | 220 | C15H24O | 1.11 | 43(100), 91, 119, 41, 105, 205, 79, 159, 69, 131, 147, 187, 177, 220 |
| 17 | 32.30 | (−)-Caryophyllene oxide | 220 | C15H24O | 0.75 | 41(100), 79, 93, 69, 109, 121, 131, 161, 187, 177 202, 220 |
| 18 | 33.88 | α-Cadinol | 222 | C15H26O | 0.08 | 95(100), 43, 121, 161, 109, 204, 71, 58, 41, 179, 222 |
| 19 | 61.78 | Otochilone | 424 | C30H48O | 0.17 | 409(100), 65, 109, 257, 95, 55, 41, 311, 81, 149, 119, 271, 245, 297, 231, 173, 161, 204, 187, 424 |
| 20 | 62.68 | Lupenone | 424 | C30H48O | 0.38 | 95(100), 109, 205, 81, 55, 121, 69, 135, 149, 189, 41, 161, 175, 424, 128, 313, 245, 355 |
| Samples | IC50 (µg/mL) | |||||
|---|---|---|---|---|---|---|
| Vero | HCT116 | SK-LU-1 | SK-MEL-2 | SiHa | U937 | |
| Leaves | >500 c | >500 d | >500 d | >500 c | >500 d | 91.3 ± 6.2 b |
| Bark | >500 c | >500 d | 273.0 ± 18.3 b | >500 c | 197.8 ± 15.5 c | 106.1 ± 7.8 c |
| Twigs | >500 c | 440.6 ± 28.7 c | > 500 d | >500 c | >500 d | 128.9 ± 2.5 d |
| Oleo-resin | 88.7 ± 4.1 a | 340.3 ± 21.1 b | 336.6 ± 6.7 c | 215.2 ± 10.9 b | 59.6 ± 1.7 b | 63.3 ± 2.1 a |
| Melphalan | 215.6 ± 3.7 b | 179.6 ± 12.2 a | 56.9 ± 0.3 a | 20.2 ± 2.1 a | 27.1 ± 0.8 a | 228.4 ± 8.8 e |
| Samples | Selective Index (SI) * | ||||
|---|---|---|---|---|---|
| HCT116 | SK-LU-1 | SK-MEL-2 | SiHa | U937 | |
| Leaves | 1.0 | 1.0 | 1.0 | 1.0 | >5.5 |
| Bark | 1.0 | >1.8 | 1.0 | >2.5 | >4.7 |
| Twigs | >1.1 | 1.0 | 1.0 | 1.0 | >3.9 |
| Oleo-resin | 0.3 | 0.3 | 0.4 | 1.5 | 1.4 |
| Melphalan | 1.2 | 3.8 | 10.7 | 8.0 | 0.9 |
| Samples | Antioxidant Capacity | Total Phenolic Content (mg GAE/g DW) | ||
|---|---|---|---|---|
| DPPH IC50 (µg/mL) | ABTS IC50 (µg/mL) | FRAP (mmole/100 g DW) | ||
| Leaves | 26.76 ± 0.25 d | 13.56 ± 0.09 c | 124.80 ± 0.19 c | 308.60 ± 4.32 b |
| Bark | 5.76 ± 0.19 b | 9.37 ± 0.03 a | 300.67 ± 2.93 b | 366.43 ± 11.52 a |
| Twigs | 16.53 ± 0.61 c | 15.46 ± 0.02 d | 102.79 ± 1.01 d | 128.29 ± 3.89 c |
| Oleo-resin | >1000 e | >1000 e | 21.02 ± 0.19 e | 15.14 ± 0.62 d |
| Trolox | 3.93 ± 0.02 a | 10.20 ± 0.10 b | 771.70 ± 11.37 a | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yongram, C.; Sungthong, B.; Puthongking, P.; Weerapreeyakul, N. Chemical Composition, Antioxidant and Cytotoxicity Activities of Leaves, Bark, Twigs and Oleo-Resin of Dipterocarpus alatus. Molecules 2019, 24, 3083. https://doi.org/10.3390/molecules24173083
Yongram C, Sungthong B, Puthongking P, Weerapreeyakul N. Chemical Composition, Antioxidant and Cytotoxicity Activities of Leaves, Bark, Twigs and Oleo-Resin of Dipterocarpus alatus. Molecules. 2019; 24(17):3083. https://doi.org/10.3390/molecules24173083
Chicago/Turabian StyleYongram, Chawalit, Bunleu Sungthong, Ploenthip Puthongking, and Natthida Weerapreeyakul. 2019. "Chemical Composition, Antioxidant and Cytotoxicity Activities of Leaves, Bark, Twigs and Oleo-Resin of Dipterocarpus alatus" Molecules 24, no. 17: 3083. https://doi.org/10.3390/molecules24173083
APA StyleYongram, C., Sungthong, B., Puthongking, P., & Weerapreeyakul, N. (2019). Chemical Composition, Antioxidant and Cytotoxicity Activities of Leaves, Bark, Twigs and Oleo-Resin of Dipterocarpus alatus. Molecules, 24(17), 3083. https://doi.org/10.3390/molecules24173083