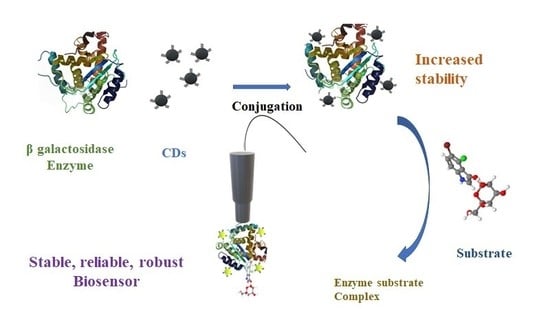
Conjugation of Carbon Dots with β-Galactosidase Enzyme: Surface Chemistry and Use in Biosensing
Abstract
:1. Introduction
2. Results and Discussion
2.1. UV-Vis Spectra of the Conjugate
2.2. Fluorescence Spectra of the Conjugate
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. Study of the Conjugate Cytotoxicity on Sea Urchin Embryos
2.5. Surface Chemistry and Spectroscopic Studies of the Conjugate
2.5.1. Surface Pressure and Surface Potential
2.5.2. X-gal Concentration Effects on the Langmuir Monolayer of β-galactosidase-CDs Conjugate
2.5.3. In Situ UV-Vis Spectrum of the Langmuir Monolayer
2.6. Monitoring the Reaction of the Conjugate and Lactose
2.6.1. Development of a Biosensing Platform
2.6.2. Cyclic Voltammetry Study
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of CDs
3.3. Conjugation of β-Galactosidase with CDs
3.4. Characterization of the Biocomposite
3.5. Immobilization of Conjugate on the Glassy-Carbon Electrode
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahmoud, M.A.; O’Neil, D.; El-Sayed, M.A. Hollow and solid metallic nanoparticles in sensing and in nanocatalysis. Chem. Mater. 2013, 26, 44–58. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal organic frameworks as versatile hosts of Au nanoparticles in heterogeneous catalysis. ACS Catal. 2017, 7, 2896–2919. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, M.; Li, C. Copper-based nanomaterials for cancer imaging and therapy. Bioconjug. Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Xie, C.; Zhen, X.; Lyu, Y.; Duan, H.; Liu, X.; Jokerst, J.V.; Pu, K. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 2017, 35, 1102. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Niu, X.; Ma, K.; Huang, P.; Grothe, J.; Kaskel, S.; Zhu, Y. Graphene quantum dots-capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and hhotothermal therapy. Small 2017, 13, 160225–160231. [Google Scholar] [CrossRef]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S.; Romeo, R.; Giofré, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.; Bhatia, S.N. Intracellular delivery of quantum dots for live cell labeling and organelle tracking. Adv. Mater. 2004, 16, 961–966. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Pons, T.; Medintz, I.L.; Mattoussi, H. Biosensing with luminescent semiconductor quantum dots. Sensors 2006, 6, 925–953. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.-J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst 2013, 138, 2506–2515. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, M.R.; Vallyathan, V. Nanoparticles: Health effects—Pros and cons. Env. Health Perspect. 2006, 114, 1818. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, A.G.; Vasisht, N.; Khare, A.R.; Sobel, R. Microencapsulation in the Food Industry: A Practical Implementation Guide; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Pollard, D.J.; Woodley, J.M. Biocatalysis for pharmaceutical intermediates: The future is now. Trends Biotechnol. 2007, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Fjerbaek, L.; Christensen, K.V.; Norddahl, B. A review of the current state of biodiesel production using enzymatic transesterification. Biotechnol. Bioeng. 2009, 102, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Sharma, S.K.; Leblanc, R.M. Biosensors based on β-galactosidase enzyme: Recent advances and perspectives. Anal. Biochem. 2017, 535, 1–11. [Google Scholar] [CrossRef]
- Wang, M.; Qi, W.; Yu, Q.; Su, R.; He, Z. Cross-linking enzyme aggregates in the macropores of silica gel: A practical and efficient method for enzyme stabilization. Biochem. Eng. J. 2010, 52, 168–174. [Google Scholar] [CrossRef]
- Wasilewski, T.; Migoń, D.; Gębicki, J.; Kamysz, W. Critical review of electronic nose and tongue instruments prospects in pharmaceutical analysis. Anal. Chim. Acta 2019, 1077, 14–29. [Google Scholar] [CrossRef]
- Altintas, Z. Biosensors and Nanotechnology: Applications in Health Care Diagnostics; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Li, Y.; Liu, W.; Sun, C.; Zheng, M.; Zhang, J.; Liu, B.; Wang, Y.; Xie, Z.; Xu, N. Hybrids of carbon dots with subunit B of ricin toxin for enhanced immunomodulatory activity. J. Colloid Interface Sci. 2018, 523, 226–233. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Rui, Y.; Li, M. Horseradish peroxidase immobilization on carbon nanodots/CoFe layered double hydroxides: Direct electrochemistry and hydrogen peroxide sensing. Biosens Bioelectron. 2015, 64, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Jana, N.R.; Jana, N.R. Inhibition of protein aggregation by iron oxide nanoparticles conjugated with glutamine-and proline-based osmolytes. ACS Appl. Nano Mater. 2018, 1, 1094–1103. [Google Scholar] [CrossRef]
- Hosseinzadeh, G.; Maghari, A.; Farniya, S.M.F.; Keihan, A.H.; Moosavi-Movahedi, A.A. Interaction of insulin with colloidal ZnS quantum dots functionalized by various surface capping agents. Mater. Sci. Eng. C 2017, 77, 836–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, O.S.; Song, H.S.; Park, T.H.; Jang, J. Conducting nanomaterial sensor using natural receptors. Chem. Rev. 2018, 119, 36–93. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Desserre, A.; Sharma, S.K.; Li, S.; Marksberry, M.H.; Chusuei, C.C.; Blackwelder, P.L.; Leblanc, R.M. Gel-like carbon dots: Characterization and their potential applications. ChemPhysChem 2017, 18, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Simchi, A.; Aashuri, H. Enzymatic biosensing by covalent conjugation of enzymes to 3D-networks of graphene nanosheets on arrays of vertically aligned gold nanorods: Application to voltammetric glucose sensing. Microchim. Acta 2018, 185, 178. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Poudel Sharma, S.; Miller, D.; Parel, J.-M.A.; Leblanc, R.M. Interfacial behavior of fumonisin B1 toxin and its degradation on the membrane. Langmuir 2019, 35, 2814–2820. [Google Scholar] [CrossRef]
- Sharma, S.K.; Seven, E.S.; Micic, M.; Li, S.; Leblanc, R.M. Surface chemistry and spectroscopic study of a cholera toxin B Langmuir monolayer. Langmuir 2018, 34, 2557–2564. [Google Scholar] [CrossRef]
- Sharma, S.K.; Li, S.; Micic, M.; Orbulescu, J.; Weissbart, D.; Nakahara, H.; Shibata, O.; Leblanc, R.M. β-Galactosidase Langmuir monolayer at air/x-gal subphase interface. J. Phys. Chem. B 2016, 120, 12279–12286. [Google Scholar] [CrossRef]
- Kissinger, P.T.; Heineman, W.R. Cyclic voltammetry. J. Chem. Edu. 1983, 60, 702–709. [Google Scholar] [CrossRef]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical sensors and biosensors. Anal. Chem. 2011, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Electrochemical immunosensor based on chitosan/conductive carbon black composite modified disposable ITO electrode: An analytical platform for p53 detection. Biosens. Bioelectron. 2018, 121, 80–89. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds CDs, conjugated CDs-enzyme are available from the authors. |















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.K.; Micic, M.; Li, S.; Hoar, B.; Paudyal, S.; Zahran, E.M.; Leblanc, R.M. Conjugation of Carbon Dots with β-Galactosidase Enzyme: Surface Chemistry and Use in Biosensing. Molecules 2019, 24, 3275. https://doi.org/10.3390/molecules24183275
Sharma SK, Micic M, Li S, Hoar B, Paudyal S, Zahran EM, Leblanc RM. Conjugation of Carbon Dots with β-Galactosidase Enzyme: Surface Chemistry and Use in Biosensing. Molecules. 2019; 24(18):3275. https://doi.org/10.3390/molecules24183275
Chicago/Turabian StyleSharma, Shiv K., Miodrag Micic, Shanghao Li, Benjamin Hoar, Suraj Paudyal, Elsayed M. Zahran, and Roger M. Leblanc. 2019. "Conjugation of Carbon Dots with β-Galactosidase Enzyme: Surface Chemistry and Use in Biosensing" Molecules 24, no. 18: 3275. https://doi.org/10.3390/molecules24183275
APA StyleSharma, S. K., Micic, M., Li, S., Hoar, B., Paudyal, S., Zahran, E. M., & Leblanc, R. M. (2019). Conjugation of Carbon Dots with β-Galactosidase Enzyme: Surface Chemistry and Use in Biosensing. Molecules, 24(18), 3275. https://doi.org/10.3390/molecules24183275