9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol
Abstract
:1. Introduction
1.1. Classification and Nomenclature of Norlignans
1.2. 9-Norlignans
1.3. Occurrence and Biological Activity
2. Results
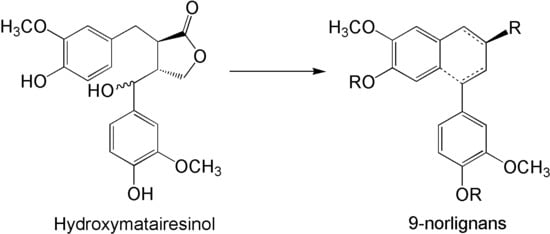
Semisynthesis of Norlignan Derivatives from Hydroxymatairesinol
3. Discussion and Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsunaga, K.; Shibya, M.; Ohizumi, Y. Imperanene, a novel phenolic compound with platelet aggregation inhibitory activity from Imperata cylindrica. J. Nat. Prod. 1995, 58, 138–139. [Google Scholar] [CrossRef]
- Kawazoe, K.; Yutani, A.; Tamemoto, K.; Yuasa, S.; Shibata, H.; Higuti, T.; Takaishi, Y. Phenylnaphthalene Compounds from the Subterranean Part of Vitex rotundifolia and Their Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus. J. Nat. Prod. 2001, 64, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Bai, R.; Yu, X.; Cao, Y.; Yin, X.; Tu, P.; Chai, X. Noralashinol A, a new norlignan from stem barks of Syringa pinnatifolia. Nat. Prod. Res. 2016, 19, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.F.; Feng, X.; Su, G.Z.; Yin, X.; Yang, X.Y.; Zhao, Y.F.; Li, W.F.; Tu, P.F.; Chai, X.Y. Noralashinol B, a norlignan with cytotoxicity from stem barks of Syringa pinnatifolia. J. Asian Nat. Prod. Res. 2017, 19, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Eklund, P.; Riska, A.; Sjöholm, R. Synthesis of R-(−)-Imperanene from the Natural Lignan Hydroxymatairesinol. J. Org. Chem. 2002, 67, 7544–7546. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; Tanimura, R.; Nishiwaki, H.; Nishi, K.; Sugahara, T.; Maruyama, M.; Ano, Y.; Akiyama, K.; Kishida, T. Enantioselective syntheses of both enantiomers of 9′-dehydroxyimperanene and 7,8-dihydro-9′-dehydroxyimperanene and the comparison of biological activity between 9-norlignans and dihydroguaiaretic acids. Bioorg. Med. Chem. Lett. 2016, 26, 3019–3023. [Google Scholar] [CrossRef] [PubMed]
- Moss, G.P. Nomenclature of Lignans and Neolignans (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1493–1523. [Google Scholar] [CrossRef] [Green Version]
- Erdtman, H.; Harmata, J. Phenolic and Terpenoid Heartwood Constituents of Libocedrus Yateensis. Phytochemistry 1979, 18, 1495–1500. [Google Scholar] [CrossRef]
- Ngadjui, B.T.; Lontsi, D.; Ayafor, J.F.; Sondengam, B.L. Pachypophyllin and pachypostaudins A and B: Three bisnorlignans from Pachypodantum staudtii. Phytochemistry 1989, 28, 231–234. [Google Scholar] [CrossRef]
- Wang, B.-G.; Ebel, R.; Wang, C.-Y.; Wray, V.; Proksch, P. New methoxylated aryltetrahydronaphthalene lignans and a norlignan from Aglaia cordata. Tetrahedron Lett. 2002, 43, 5783–5787. [Google Scholar] [CrossRef]
- Kawazoe, K.; Yutani, A.; Takaishi, Y. Aryl naphthalenes norlignans from Vitex rotundifolia. Phytochemistry 1999, 52, 1657–1659. [Google Scholar] [CrossRef]
- Sun, K.; Li, X.; Li, W.; Liu, J.-M.; Wang, J.-H.; Yi, S. A new nor-lignan from the seeds of Descurainia sophia. Nat. Prod. Res. 2006, 20, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.M.; Fouad, M.A.; Matsunami, K.; Kamel, M.S.; Otsuka, H. A new norlignan glycoside from Cestrum diurnum L. Arkivoc 2007, 4, 63–70. [Google Scholar] [CrossRef]
- Susplugas, S.; Van Hung, N.; Bignon, J.; Thoison, O.; Kruczynski, A.; Sévenet, T.; Guéritte, F. Cytotoxic Arylnaphthalene Lignans from a Vietnamese Acanthaceae, Justicia patentiflora. J. Nat. Prod. 2005, 68, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Chen, C.-C.; Hsu, F.-L.; Chen, C.-F. Tannins, Flavonol Sulfonates, and a Norlignan from Phyllantus virgatus. J. Nat. Prod. 1998, 61, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Rishmann, M.; Mues, R.; Geiger, H.; Laas, H.J.; Eicher, T. Isolation and synthesis of 6,7-dihydroxy-4-(3,4-dihydroxyphenyl)naphthalene-2-carboxylic acid from Pellia epiphylla. Phytochemistry 1989, 28, 867–869. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Dellagreca, M.; Fiorentino, A.; Golino, A.; Minaco, P.; Zarrelli, A. Isolation and characterization of new lignans from the leaves of Cestrum parqui. Nat. Prod. Res. 2006, 20, 293–298. [Google Scholar] [CrossRef]
- Ekman, R.; Sjöholm, R.T.; Sjöholm, R. A Degraded Lignan from Alkaline Hydrolysis of Norway Spruce Root Extractives. Finn. Chem. Lett. 1979, 4, 126–128. [Google Scholar]
- Takara, K.; Iwasaki, H.; Ujihara, K.; Wada, K. Human Tyrosinase Inhibitor in Rum Distillate Wastewater. J. Oleo Sci. 2008, 57, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Lacret, R.; Varela, R.M.; Molinillo, J.M.G.; Nogueiras, C.; Macías, F.A. Tectonoelins, new norlignans from a bioactive extract of Tectona grandis. Phytochem. Lett. 2012, 5, 382–385. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Huang, J.; Gong, Z.; Tian, Z.-Q. A Novel Norlignan and a Novel Phenylpropanoid from Peperomia tetraphylla. Helv. Chim. Acta 2007, 90, 2222–2226. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Tong, A.P.; Huang, J. Two New Norlignans and a New Lignanamide from Peperomia tetraphylla. Chem. Biodivers. 2012, 9, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zeng, Y.H.; Osman, K.; Shinde, K.; Rahman, M.; Gibbons, S.; Mu, Q. Norlignans, Acylphloroglucinols, and a Dimeric Xanthone from Hypericum chinense. J. Nat. Prod. 2010, 73, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.-Q.; Tao, S.-H.; Yi, Z.-B.; Guo, L.-B.; Xie, Y.-F.; Chen, Y.-F. Four New Compounds from Pouzolzia zeylanica (L.) Benn. Var. Microphylla. Heterocycles 2015, 91, 1926–1936. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, Y.-G.; Zhang, Y.; Li, T.; Li, H.-L.; Yan, S.-K.; Jin, H.-Z.; Zhang, W.-D. New norlignans and flavonoids of Dysosma versipellis. Phytochem. Lett. 2016, 16, 75–81. [Google Scholar] [CrossRef]
- Takahashi, K.; Ogiyama, K. Phenols of discolored sugi (Cryptomeria japonica D. Don) sapwood II. Norlignans of discolored sugi sapwood collected in the Kyushu region. Mokuzai Gakkaishi 1985, 31, 28–38. [Google Scholar]
- Noguchi, T.; Ohtani, Y.; Sameshima, K. Static defense components for sugi butt-rot disease. Trans. Mater. Res. Soc. Jpn. 2004, 29, 2479–2482. [Google Scholar]
- Chen, T.-H.; Liau, B.-C.; Wang, S.-Y.; Jong, T.-T. Isolation and cytotoxicity of the lignanoids from Chamaecyparis formosensis. Planta Med. 2008, 74, 1806–1811. [Google Scholar] [CrossRef]
- Mathouet, H.; Elomri, A.; Lameiras, P.; Daich, A.; Vérité, P. An alkaloid, two conjugate sesquiterpenes and a phenylpropanoid from Pachypodanthium confine Engl. and Diels. Phytochemistry 2007, 68, 1813–1818. [Google Scholar] [CrossRef]
- Lou, Z.-H.; Li, H.-M.; Gao, L.-H.; Li, R.-T. Antioxidant lignans from the seeds of Vitex negundo var. cannabifolia. J. Asian Nat. Prod. Res. 2014, 16, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.-U.; Malik, A.; Anis, I.; Khan, S.B.; Ahmed, E.; Ahmed, Z.; Nawaz, S.A.; Choudhary, M.I. Enzymes Inhibiting Lignans from Vitex negundo. Chem. Pharm. Bull. 2004, 52, 1269–1272. [Google Scholar]
- Haq, A.-U.; Malik, A.; Khan, M.T.H.; Haq, A.-U.; Khan, S.B.; Ahmad, A.; Choudhary, M.I. Tyrosinase inhibitory lignans from the methanol extract of the roots of Vitex negundo Linn. And their structure-activity relationship. Phytomedicine 2006, 13, 255–260. [Google Scholar] [CrossRef]
- Yamasaki, T.; Kawabata, T.; Masuoka, C.; Kinjo, J.; Ikeda, T.; Nohara, T.; Ono, M. Two new lignan glucosides from the fruit of Vitex cannabifolia. J. Nat. Med. 2008, 62, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-H.; Huo, S.R.N.; Bao, Y.P.; Ao, W.L.J. Chemical constituents of Syringa pinnatifolia and its chemotaxonomic study. Chem. Nat. Compd. 2018, 54, 435–438. [Google Scholar] [CrossRef]
- Ono, M.; Nishida, Y.; Masuoka, C.; Li, J.-C.; Okawa, M.; Ikeda, T.; Nohara, T. Lignan Derivatives and a Norditerpene from the seeds of Vitex negundo. J. Nat. Prod. 2004, 67, 2073–2075. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, M.A.; Haq, A.U.; Choudhary, M.I.; Malik, A.; Ahmad, S. Chymotrypsin inhibition studies on the lignans from Vitex negundo Linn. J. Enzyme Inhib. Med. Chem. 2008, 23, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.; Neufeld, N. Animal Feed Additive Containing Diurnoside and/or Cestrumoside. PCT Int. Appl. WO 2017129732 A1 20170803, 3 August 2017. [Google Scholar]
- Cabanillas, B.J.; Le Lamer, A.-C.; Castillo, D.; Arevalo, J.; Rojas, R.; Odonne, G.; Bourdy, G.; Moukarzel, B.; Sauvain, M.; Fabre, N. Caffeic Acid Esters and Lignans from Piper sanguineispicum. J. Nat. Prod. 2010, 73, 1884–1890. [Google Scholar] [CrossRef]
- Huang, R.L.; Huang, Y.L.; Ou, J.C.; Chen, C.C.; Hsu, F.L.; Chang, C. Screening of 25 Compounds isolated from Phyllantus Species for Anti-Human Hepatitis B Virus In Vitro. Phytother. Res. 2003, 17, 449–453. [Google Scholar] [CrossRef]
- Tazaki, H.; Adam, K.-P.; Becker, H. Five lignan derivatives from in vitro cultures of liverwort Jamesoniella autumnalis. Phytochemistry 1995, 40, 1671–1675. [Google Scholar] [CrossRef]
- Martini, U.; Zapp, J.; Becker, H. Lignans from the liverwort Bazzania Trilobata. Phytochemistry 1998, 49, 1139–1146. [Google Scholar] [CrossRef]
- Sanderson, S. Phytochemische Untersuchungen der Ledermoose Jamesoniella rubricaulis (Nees) Grolle und Lepidozia reptans (L.) Dum. Ph.D. Thesis, Universität des Saarlandes, Saarbrucken, Germany, 1995. [Google Scholar]
- Cullman, F.; Schmidt, A.; Schuld, F.; Trennheuser, M.L.; Becker, H. Lignans from the liverworts Lepidozia incurvata, Chiloscyphus polyanthos and Jungermannia exsertifolia ssp. cordifolia. Phytochemistry 1999, 52, 1647–1650. [Google Scholar] [CrossRef]
- Wróbel, A.; Eklund, P.; Bobrowska-Hägerstrand, M.; Hägerstrand, H. Lignans and norlignans Inhibit Multidrug Resistance Protein 1(MRP1/ABCC1)-mediated Transport. Anticancer Res. 2010, 30, 4423–4428. [Google Scholar] [PubMed]
- Aehle, E.; Müller, U.; Eklund, P.C.; Willför, S.M.; Sippl, W.; Dräger, B. Lignans as food constituents with estrogen and antiestrogen activity. Phytochemistry 2011, 72, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Plumed-Ferrer, C.; Väkeväinen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J.-E.; Eklund, P.; Willför, S.; Alakomi, H.-L.; Saarela, M.; et al. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int. J. Food Microbiol. 2013, 164, 99–107. [Google Scholar] [CrossRef] [PubMed]




| Compound | Number | Occurrence | Bioactivity | References |
|---|---|---|---|---|
| Yateresinol | 1 | Libocedrus yateensis, Cryptomerica japonica | Antifungal | [8,26] |
| Pachypostaudin A | 2 | Pachypodanthium staudtii, Pachypodanthium confine | [9,29] | |
| Pachypostaudin B | 3 | |||
| Aglacin H | 4 | Aglaia cordata | [10] | |
| Vitrofolal A | 5 | Vitex rotundifolia, Vitex negundo, Vitex cannabifolia, Syringa pinnatifolia | Antibacterial, antioxidant, cholinesterase inhibition, tyrosinase inhibitory effects, chymotrypsin inhibitory effects | [2,11,30,31,32,33,34,36] |
| Vitrofolal B | 6 | |||
| Vitrofolal E | 7 | |||
| Vitrofolal F | 8 | |||
| Descuraic Acid | 9 | Descurainia Sophia | [12] | |
| Cestrumoside | 10 | Cestrum diurnum L. | [13] | |
| (−)-Justiflorinol | 11 | Justicia patentiflora, Piper sanguineispicum | [14,38] | |
| (+)-Virgatyne | 12 | Phyllantus virgatus | [15] | |
| Arylnaphthalene | 13 | Pellia epiphylla, Jamesoniella autumnalis, Bazzania trilobata, Lepidozia reptans, Lepidozia incurvata, Chiloscyphus polyanthos, Jungermannia exsertifolia ssp. cordifolia | [16,40,41,42,43] | |
| Lactone | 14 | Cestrum parqui | Phytotoxic effects | [17] |
| Compound | 15 | Picea abies | [18] | |
| (+)-Imperanene | 16 | Imperata cylindrica, sugarcane rum distillate | Platelet aggregation inhibitory effect, tyrosinase inhibitory effect | [1,19] |
| 3-Methoxy-Imperanene | 18 | Sugarcane rum distillate | Tyrosinase inhibitory effect | [19] |
| Noralashinol C | 19 | Syringa pinnatifolia | [3,4] | |
| Noralashinol A | 20 | |||
| Tectonoelin A | 21 | Tectona grandis | Herbicidal activity | [20] |
| Tectonoelin B | 22 | |||
| Peperoteraphin | 23 | Peperomia tetraphylla | Cytotoxic effect | [21,22] |
| Cyclobutane carboxylate | 24 | |||
| Cyclobutane carboxylate | 25 | |||
| Hyperione A | 26 | Hypericum chinense | [23] | |
| Hyperione B | 27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eklund, P.; Raitanen, J.-E. 9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol. Molecules 2019, 24, 220. https://doi.org/10.3390/molecules24020220
Eklund P, Raitanen J-E. 9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol. Molecules. 2019; 24(2):220. https://doi.org/10.3390/molecules24020220
Chicago/Turabian StyleEklund, Patrik, and Jan-Erik Raitanen. 2019. "9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol" Molecules 24, no. 2: 220. https://doi.org/10.3390/molecules24020220
APA StyleEklund, P., & Raitanen, J. -E. (2019). 9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol. Molecules, 24(2), 220. https://doi.org/10.3390/molecules24020220