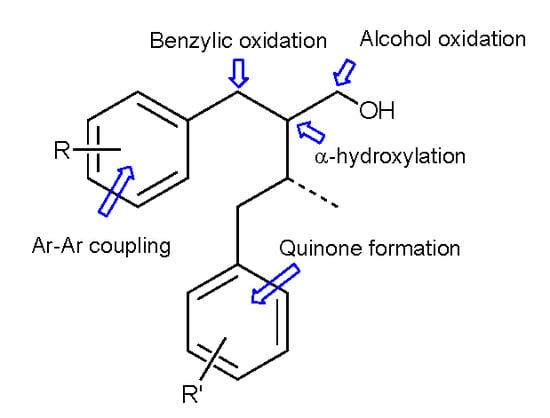
Oxidative Transformations of Lignans
Abstract
:1. Introduction
2. Non-Metal-Mediated Oxidations
2.1. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ)
2.2. Meta-Chloroperoxybenzoic Acid (mCPBA)
2.3. Oxidations by Peroxyl Radical
2.4. Azo Compounds (AAPH, AIBN and ABTS)
2.5. 2,2-Diphenyl-1-picrylhydrazyl (DPPH)
2.6. 2,2,6,6-Tetramethyl-1-piperidinyloxy (TEMPO)
2.7. Hypervalent Iodine Reagents
2.7.1. [Bis(acetoxy)iodo]benzene (BAIB or PIDA) and [Bis(trifluoroacetoxy)iodo]benzene (PIFA)
2.7.2. 3-Iodobenzoic Acid (IBX)
2.7.3. Dess-Martin Periodinane (DMP)
2.7.4. Sodium Periodate (NaIO4)
2.8. N-Bromosuccinimide (NBS)
2.9. Dimethyldioxirane (DMDO or DMD)
2.10. Nitrobenzene
3. Metal-Mediated Oxidations
3.1. Chromium (VI) Oxidations
3.2. Palladium and Gold Mediated Oxidations
3.3. Molybdenium Mediated Oxidations
3.4. Vanadium, Thallium and Ruthenium Oxidations
3.5. Methyl Trioxo-Rhenium (MTO) Catalyzed Oxidations
3.6. Other Metal Mediated Lignan Oxidations
4. Other Oxidation Methods
4.1. Enzymatic Oxidations
4.2. Electrochemical Oxidations
4.3. Photooxidations
5. Conclusions
Funding
Conflicts of Interest
References
- Zhang, J.; Chen, J.; Liang, Z.; Zhao, C. New Lignans and Their Biological Activities. Chem. Biodivers. 2014, 11, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef] [PubMed]
- Harmatha, J.; Dinan, L. Biological activities of lignans and stilbenoids associated with plant-insect chemical interactions. Phytochem. Rev. 2003, 2, 321–330. [Google Scholar] [CrossRef]
- Talaei, M.; Pan, A. Role of phytoestrogens in prevention and management of type 2 diabetes. World J. Diabetes 2015, 6, 271–283. [Google Scholar] [CrossRef]
- Deo, S.; Utane, R.; Khubalkar, R.; Thombre, S. Extraction and isolation, synthesis, physiological activity of 1-phenyl naphthalene and its derivatives: A review. Pharma Innov. J. 2017, 6, 21–30. [Google Scholar]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adlercreutz, H. Lignans and Human Health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Waśkiewicz, A.; Zujko, M.E.; Szcześniewska, D.; Stepaniak, U.; Pająk, A.; Drygas, W. Are Total and Individual Dietary Lignans Related to Cardiovascular Disease and Its Risk Factors in Postmenopausal Women? A Nationwide Study. Nutrients 2018, 10, 865. [Google Scholar] [CrossRef]
- Kiyama, R. Biological effects induced by estrogenic activity of lignans. Trends Food Sci. Technol. 2016, 54, 186–196. [Google Scholar] [CrossRef]
- Bobe, G.; Murphy, G.; Albert, P.S.; Sansbury, L.B.; Lanza, E.; Schatzkin, A.; Cross, A.J. Dietary lignan and proanthocyanidin consumption and colorectal adenoma recurrence in the Polyp Prevention Trial. Int. J. Cancer 2012, 130, 1649–1659. [Google Scholar] [CrossRef]
- Hearon, W.M.; MacGregor, W.S. The Naturally Occurring Lignans. Chem. Rev. 1955, 55, 957–1068. [Google Scholar] [CrossRef]
- Rao, C. Chemistry of Lignans; Andhra University Press: Visakhapatnam, India, 1978. [Google Scholar]
- Ayres, D.C.; Loike, J.D. Lignans: Chemical, Biological, and Clinical Properties; Phillipson, J.D., Ayres, D.C., Baxter, H., Eds.; Cambridge University Press: Cambridge, UK, 1990; ISBN 0521304210. [Google Scholar]
- Whiting, D.A. Lignans, neolignans, and related compounds. Nat. Prod. Rep. 1987, 4, 499–525. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.S. Lignans, neolignans and related compounds. Nat. Prod. Rep. 1999, 16, 75–96. [Google Scholar] [CrossRef]
- Kariyappa, A.K.; Ramachandrappa, R.K.; Seena, S. Lignans: Insight to Chemistry and Pharmacological Applications-An Overview. Chem Sci Rev Lett. 2015, 4, 1157–1165. [Google Scholar]
- Ward, R.S. Recent Advances in the Chemistry of Lignans. Stud. Nat. Prod. Chem. 2000, 24, 739–798. [Google Scholar] [CrossRef]
- Pelter, A.; Ward, R.S.; Venkateswarlu, R.; Kamakshi, C. Oxidative transformations of lignans - reactions of dihydrocubebin and a derivative with DDQ. Tetrahedron 1991, 47, 1275–1284. [Google Scholar] [CrossRef]
- Venkateswarlu, R.; Kamakshi, C.; Moinuddin, S.G.; Subhash, P.V.; Ward, R.S.; Pelter, A.; Hursthouse, M.B.; Light, M.E. Transformations of lignans, Part V. Reactions of DDQ with a gmelinol hydrogenolysis product and its derivatives. Tetrahedron 1999, 55, 13087–13108. [Google Scholar] [CrossRef]
- Ward, R.S.; Hughes, D.D. Oxidative cyclisation of cis- and trans-2,3-dibenzylbutyrolactones using phenyl iodonium bis(trifluoroacetate) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. Tetrahedron 2001, 57, 5633–5639. [Google Scholar] [CrossRef]
- Charlton, J.L.; Chee, G.-L. Asymmetric synthesis of lignans using oxazolidinones as chiral auxiliaries. Can. J. Chem. 1997, 75, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- Magoulas, G.; Papaioannou, D.; Magoulas, G.E.; Papaioannou, D. Bioinspired Syntheses of Dimeric Hydroxycinnamic Acids (Lignans) and Hybrids, Using Phenol Oxidative Coupling as Key Reaction, and Medicinal Significance Thereof. Molecules 2014, 19, 19769–19835. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, P.; Venkateswarlu, S.; Viswanatham, K.N. Oxidative aryl-aryl, aryl-benzyl coupling of lignans-reactions of phyllanthin and haloderivatives with TTFA, DDQ, Li/THF: synthesis of dibenzocyclooctadiene system and phyltetralin. Tetrahedron 1991, 47, 8277–8284. [Google Scholar] [CrossRef]
- Pelter, A.; Ward, R.S.; Jones, D.M.; Maddocks, P. Asymmetric synthesis of lignans of the dibenzylbutanediol and tetrahydrodibenzocyclooctene series. J. Chem. Soc. Perkin Trans. 1 1993, 2631–2637. [Google Scholar] [CrossRef]
- Pelter, A.; Ward, R.S.; Jones, D.M.; Maddocks, P. Asymmetric syntheses of lignans of the dibenzylbutyrolactone, dibenzylbutanediol, aryltetraun and dibenzocyclooctadiene series. Tetrahedron: Asymmetry 1992, 3, 239–242. [Google Scholar] [CrossRef]
- Chattopadhyay, S.K.; Rao, K.V. Chemistry of saururus cernuus IV: cyclooctadiene systems derived from austrobailignan-5. Tetrahedron 1987, 43, 669–678. [Google Scholar] [CrossRef]
- Venkateswarlu, R.; Kamakshi, C.; Moinuddin, S.G.; Subhash, P.V.; Ward, R.S.; Pelter, A.; Coles, S.J.; Hursthouse, M.B.; Light, M.E. Transformations of lignans. Part 4: Oxidative and reductive rearrangements of dibenzocyclooctadiene and spirodienone lignans. Tetrahedron 2001, 57, 5625–5632. [Google Scholar] [CrossRef]
- Tomioka, K.; Ishiguro, T.; Koga, K. First asymmetric total synthesis of (+)-steganacin determination of absolute stereochemistry. Tetrahedron Lett. 1980, 21, 2973–2976. [Google Scholar] [CrossRef]
- Chang, J.; Xie, J. Synthesis of some new lignans and the mechanism of intramolecular nonphenolic oxidative coupling of aromatic compounds. Sci. China Ser. B Chem. 2000, 43, 323–330. [Google Scholar] [CrossRef]
- Tilve, S.G.; Torney, P.S.; Patre, R.E.; Kamat, D.P.; Srinivasan, B.R.; Zubkov, F.I. Domino Wittig-Diels Alder reaction: Synthesis of carbazole lignans. Tetrahedron Lett. 2016, 57, 2266–2268. [Google Scholar] [CrossRef]
- Ward, R.S.; Satyanarayana, P.; Gopala Rao, B.V. Reactions of Aryltetralin Lignans with DDQ—An Example of DDQ Oxidation of Allylic Ether Groups. Tetrahedron Lett. 1981, 22, 3021–3024. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Arimoto, M.; Tanoguchi, M.; Ishida, T.; Inoue, M. Studies on the constituents of the seeds of Hernandia ovigera L. III. Structures of two new lignans. Chem. Pharm. Bull. (Tokyo) 1982, 30, 3212–3218. [Google Scholar] [CrossRef]
- Eklund, P.C.; Sjöholm, R.E. Oxidative transformation of the natural lignan hydroxymatairesinol with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. Tetrahedron 2003, 59, 4515–4523. [Google Scholar] [CrossRef]
- Taskinen, A.; Eklund, P.; Sjöholm, R.; Hotokka, M. The molecular structure and some properties of hydroxymatairesinol. An ab initio study. J. Mol. Struct. THEOCHEM 2004, 677, 113–124. [Google Scholar] [CrossRef]
- Ishiguro, T.; Mizuguchi, H.; Tomioka, K.; Koga, K. Stereoselective reactions. VIII. Stereochemical requirement for the benzylic oxidation of lignan lactone. A highly selective synthesis of the antitumor lignan lactone steganacin by the oxidation of stegane. Chem. Pharm. Bull. 1985, 33, 609–617. [Google Scholar] [CrossRef]
- Ward, R.S.; Pelter, A.; Jack, I.R.; Satyanarayana, P.; Rao, B.V.G.; Subrahmanyam, P. Reactions of paulownin, gmelinol and gummadiol with 2,5-dichloro-5,6-dicyanobenzoquinone. Tetrahedron Lett. 1981, 22, 4111–4114. [Google Scholar] [CrossRef]
- Ward, R.S.; Pelter, A.; Venkateswarlu, R.; Kamakshi, C.; Subhash, P.V.; Moinuddin, S.G.; Hursthouse, M.B.; Coles, S.J.; Hibbs, D.E. Transformations of lignans, part IV. Acid-catalysed rearrangements of gmelinol with BF3-etherate and study of a product with a unique lignan skeleton formed by further oxidation with DDQ. Tetrahedron 1999, 55, 13071–13086. [Google Scholar] [CrossRef]
- Chang, J.B.; Xie, J.X. Synthesis of New Schizandrin Analogues. Chinese Chem. Lett. 2001, 12, 667–670. [Google Scholar]
- Orito, K.; Sasaki, T.; Suginome, H. Photoinduced molecular transformations. 158. A total synthesis of (.+-.)-methyl piperitol: An unsymmetrically substituted 2,6-diaryl-3,7-dioxabicyclo [3.3.0] octane lignan. J. Org. Chem. 1995, 60, 6208–6210. [Google Scholar] [CrossRef]
- Suginome, H.; Orito, K.; Yorita, K.; Ishikawa, M.; Shimoyama, N.; Sasaki, T. Photoinduced Molecular Transformations. 157. A New Stereo- and Regioselective Synthesis of 2,6-Diaryl-3,7 dioxabicyclo[3.3.0]octane Lignans Involving a β-Scission of Alkoxyl Radicals as the Key Step. New Total Syntheses of (±)-Sesamin, (±)-Eudesmin, and. (+)-Yangambin. J. Org. Chem. 1995, 60, 3052–3064. [Google Scholar] [CrossRef]
- Sato, Y.; Tamura, T.; Mori, M. Arylnaphthalene Lignans through Pd-Catalyzed[2+2+2] Cocyclization of Arynes and Diynes: Total Synthesis of Taiwanins C and E. Angew. Chem. Int. Ed. 2004, 43, 2436–2440. [Google Scholar] [CrossRef]
- Peng, Y.; Xiao, J.; Xu, X.-B.; Duan, S.-M.; Ren, L.; Shao, Y.-L.; Wang, Y.-W. Stereospecific Synthesis of Tetrahydronaphtho[2,3-b]furans Enabled by a Nickel-Promoted Tandem Reductive Cyclization. Org. Lett. 2016, 18, 5170–5173. [Google Scholar] [CrossRef]
- Sang, S.; Tian, S.; Wang, H.; Stark, R.E.; Rosen, R.T.; Yang, C.S.; Ho, C.-T. Chemical studies of the antioxidant mechanism of tea catechins: radical reaction products of epicatechin with peroxyl radicals. Bioorg. Med. Chem. 2003, 11, 3371–3378. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y. Comparative study of the alkyl and peroxy radical scavenging activities of polyphenols. Chemosphere 2006, 62, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, C.; Daquino, C.; Tringali, C.; Amorati, R. Reaction of benzoxanthene lignans with peroxyl radicals in polar and non-polar media: Cooperative behaviour of OH groups. Org. Biomol. Chem. 2013, 11, 4291–4294. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Akiyama, J.; Fujimoto, A.; Yamauchi, S.; Maekawa, T.; Sone, Y. Antioxidation reaction mechanism studies of phenolic lignans, identification of antioxidation products of secoisolariciresinol from lipid oxidation. Food Chem. 2010, 123, 442–450. [Google Scholar] [CrossRef]
- Betigeri, S.; Thakur, A.; Raghavan, K. Use of 2,2′-Azobis(2-Amidinopropane) Dihydrochloride as a Reagent Tool for Evaluation of Oxidative Stability of Drugs. Pharm. Res. 2005, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2011, 1. [Google Scholar] [CrossRef]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef]
- Werber, J.; Wang, Y.J.; Milligan, M.; Li, X.; Ji, J.A. Analysis of 2,2’-Azobis (2-Amidinopropane) Dihydrochloride Degradation and Hydrolysis in Aqueous Solutions. J. Pharm. Sci. 2011, 100, 3307–3315. [Google Scholar] [CrossRef]
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Muir, A.D.; Westcott, N.D.; Krol, E.S. Antioxidant capacity of flaxseed lignans in two model systems. J. Am. Oil Chem. Soc. 2006, 83, 835. [Google Scholar] [CrossRef]
- Kang, M.-H.; Naito, M.; Sakai, K.; Uchida, K.; Osawa, T. Mode of action of sesame lignans in protecting lowdensity lipoprotein against oxidative damage in vitro. Life Sci. 1999, 66, 161–171. [Google Scholar] [CrossRef]
- Hodaj, E.; Tsiftsoglou, O.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Lignans and indole alkaloids from the seeds of Centaurea vlachorum Hartvig (Asteraceae), growing wild in Albania and their biological activity. Nat. Prod. Res. 2017, 31, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, P.A.S.; Aluko, R.E. Anti-carcinogenic and anti-metastatic effects of flax seed lignan secolariciresinol diglucoside (SDG). Discov. Phytomedicine 2015, 2, 12–17. [Google Scholar] [CrossRef]
- Hosseinian, F.F.H. Antioxidant Properties of Flaxseed Lignans Using In Vitro Model Systems. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2006. [Google Scholar]
- Hosseinian, F.S.; Muir, A.D.; Westcott, N.D.; Krol, E.S. AAPH-mediated antioxidant reactions of secoisolariciresinol and SDG. Org. Biomol. Chem. 2007, 5, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, N.S.; Aidhen, I.S. Radical mediated intramolecular arylation using tributyltinhydride/aibn: A formal synthesis of steganone. Tetrahedron Lett. 1988, 29, 2987–2988. [Google Scholar] [CrossRef]
- Fischer, J.; Reynolds, A.J.; Sharp, L.A.; Sherburn, M.S. Radical Carboxyarylation Approach to Lignans. Total Synthesis of (−)-Arctigenin, (−)-Matairesinol, and Related Natural Products. Org. Lett. 2004, 6, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-X.; Bai, M.; Zhou, L.; Lou, L.-L.; Liu, Q.-B.; Zhang, Y.; Li, L.-Z.; Song, S.-J. Food Byproducts as a New and Cheap Source of Bioactive Compounds: Lignans with Antioxidant and Anti-inflammatory Properties from Crataegus pinnatifida Seeds. J. Agric. Food Chem. 2015, 63, 7252–7260. [Google Scholar] [CrossRef]
- Chen, J.-J.; Wei, H.-B.; Xu, Y.-Z.; Zeng, J.; Gao, K. Antioxidant Lignans from the Roots of Vladimiria muliensis. Planta Med. 2013, 79, 1470–1473. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.-J.; Chen, H.-Y.; Hung, Y.-C.; Chen, T.-Y.; Lee, M.-Y.; Yu, S.-C.; Chen, Y.-H.; Chuang, I.-C.; Wu, T.-S. Therapeutic window for cinnamophilin following oxygen–glucose deprivation and transient focal cerebral ischemia. Exp. Neurol. 2009, 217, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Schafberg, M.; Crișan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Szabo, M.; Idiţoiu, C.; Chambre, D.; Lupea, A. Improved DPPH determination for antioxidant activity spectrophotometric assay. Chem. Pap. 2007, 61, 214–216. [Google Scholar] [CrossRef]
- Yamauchi, S.; Masuda, T.; Sugahara, T.; Kawaguchi, Y.; Ohuchi, M.; Someya, T.; Akiyama, J.; Tominaga, S.; Yamawaki, M.; Kishida, T.; Akiyama, K.; Maruyama, M. Antioxidant Activity of Butane Type Lignans, Secoisolariciresinol, Dihydroguaiaretic Acid, and 7,7′-Oxodihydroguaiaretic Acid. Biosci. Biotechnol. Biochem. 2008, 72, 2981–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeds, A.I.; Eklund, P.C.; Monogioudi, E.; Willför, S.M. Chemical characterization of polymerized products formed in the reactions of matairesinol and pinoresinol with the stable radical 2,2-diphenyl-1-picrylhydrazyl. Holzforschung 2012, 66, 283–294. [Google Scholar] [CrossRef]
- Lei, M.; Hu, R.-J.; Wang, Y.-G. Mild and selective oxidation of alcohols to aldehydes and ketones using NaIO4/TEMPO/NaBr system under acidic conditions. Tetrahedron 2006, 62, 8928–8932. [Google Scholar] [CrossRef]
- Venkateswarlu, R.; Kamakshi, C.; Subhash, P.V.; Moinuddin, S.G.A.; Rama Sekhara Reddy, D.; Ward, R.S.; Pelter, A.; Gelbrich, T.; Hursthouse, M.B.; Coles, S.J.; et al. Transformations of lignans. Part 11: Oxidation of diphyllin with hypervalent iodine reagents and reductive reactions of a resulting 1-methoxy-1-aryl-4-oxonaphthalene lactone. Tetrahedron 2006, 62, 4463–4473. [Google Scholar] [CrossRef]
- Pelter, A.; Ward, R.S.; Abd-El-Ghani, A. Preparation of dibenzocyclooctadiene lignans and spirodienones by hypervalent iodine oxidation of phenolic dibenzylbutyrolactones. J. Chem. Soc. Perkin Trans. 1 1992, 2249–2251. [Google Scholar] [CrossRef]
- Ward, R.S.; Pelter, A.; Abd-El-Ghani, A. Preparation of tetrahydrodibenzocyclooctene lignans and spirodienones by hypervalent iodine oxidation of phenolic dibenzylbutyrolactones. Tetrahedron 1996, 52, 1303–1336. [Google Scholar] [CrossRef]
- Pelter, A.; Satchwell, P.; Ward, R.S.; Blake, K. Effective, direct biomimetic synthesis of dibenzocyclooctene lignans by hypervalent iodine oxidation of phenolic dibenzylbutyrolactones. J. Chem. Soc. Perkin Trans. 1 1995, 2201–2202. [Google Scholar] [CrossRef]
- Ward, R.S. Different strategies for the chemical synthesis of lignans. Phytochem. Rev. 2003, 2, 391–400. [Google Scholar] [CrossRef]
- Albertson, A.K.F.; Lumb, J.P. A bio-inspired total synthesis of tetrahydrofuran lignans. Angew. Chem. Int. Ed. 2015, 54, 2204–2208. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Barontini, M.; Mosesso, P.; Pepe, G.; Willför, S.M.; Sjöholm, R.E.; Eklund, P.C.; Saladino, R. A selective de-O-methylation of guaiacyl lignans to corresponding catechol derivatives by 2-iodoxybenzoic acid (IBX). The role of the catechol moiety on the toxicity of lignans. Org. Biomol. Chem. 2009, 7, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, A.; Pouységu, L.; Depernet, D.; François, B.; Quideau, S. A Stabilized Formulation of IBX (SIBX) for Safe Oxidation Reactions Including a New Oxidative Demethylation of Phenolic Methyl Aryl Ethers. Org. Lett. 2003, 5, 2903–2906. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; RajanBabu, T.V. Conformation and reactivity in dibenzocyclooctadienes (DBCOD). A general approach to the total synthesis of fully substituted DBCOD lignans via borostannylative cyclization of α,ω-diynes. Chem. Sci. 2013, 4, 3979. [Google Scholar] [CrossRef]
- Reddel, J.C.T.; Lutz, K.E.; Diagne, A.B.; Thomson, R.J. Stereocontrolled Syntheses of Tetralone- and Naphthyl-Type Lignans by a One-Pot Oxidative [3,3] Rearrangement/Friedel-Crafts Arylation. Angew. Chem. Int. Ed. 2014, 53, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Luckorse, S.; Budakoti, A.; Puranik, V.G. Synthesis of optically pure 2,3,4-trisubstituted tetrahydrofurans via a two-step sequential Michael-Evans aldol cyclization strategy: total synthesis of (+)-magnolone. Tetrahedron Lett. 2010, 51, 2975–2978. [Google Scholar] [CrossRef]
- Tohma, H.; Kita, Y. Hypervalent Iodine Reagents for the Oxidation of Alcohols and Their Application to Complex Molecule Synthesis. Adv. Synth. Catal. 2004, 346, 111–124. [Google Scholar] [CrossRef]
- Koprowski, M.; Bałczewski, P.; Owsianik, K.; Różycka-Sokołowska, E.; Marciniak, B. Total synthesis of (±)-epithuriferic acid methyl ester via Diels–Alder reaction. Org. Biomol. Chem. 2016, 14, 1822–1830. [Google Scholar] [CrossRef]
- Jung, E.-K.; Dittrich, N.; Pilkington, L.I.; Rye, C.E.; Leung, E.; Barker, D. Synthesis of aza-derivatives of tetrahydrofuran lignan natural products. Tetrahedron 2015, 71, 9439–9456. [Google Scholar] [CrossRef]
- Meresse, P.; Magiatis, P.; Bertounesque, E.; Monneret, C. Synthesis and antiproliferative activity of retroetoposide. Bioorg. Med. Chem. Lett. 2003, 13, 4107–4109. [Google Scholar] [CrossRef]
- Meresse, P.; Monneret, C.; Bertounesque, E. Synthesis of podophyllotoxin analogues: δ-lactone-containing picropodophyllin, podophyllotoxin and 4′-demethyl-epipodophyllotoxin derivatives. Tetrahedron 2004, 60, 2657–2671. [Google Scholar] [CrossRef]
- Roulland, E.; Magiatis, P.; Arimondo, P.; Bertounesque, E.; Monneret, C. Hemi-synthesis and Biological Activity of New Analogues of Podophyllotoxin. Bioorg. Med. Chem. 2002, 10, 3463–3471. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Scott, A.J.; Turner, C.I.; Sherburn, M.S. The Intramolecular Carboxyarylation Approach to Podophyllotoxin. J. Am. Chem. Soc. 2003, 125, 12108–12109. [Google Scholar] [CrossRef] [PubMed]
- Monovich, L.G.; Le Huérou, Y.; Rönn, M.; Molander, G.A. Total synthesis of (-)-steganone utilizing a samarium(II) iodide promoted 8-endo ketyl-olefin cyclization. J. Am. Chem. Soc. 2000, 122, 52–57. [Google Scholar] [CrossRef]
- Davidson, S.J.; Pearce, A.N.; Copp, B.R.; Barker, D. Total Synthesis of (−)-Bicubebin A, B, (+)-Bicubebin C and Structural Reassignment of (−)-cis-Cubebin. Org. Lett. 2017, 19, 5368–5371. [Google Scholar] [CrossRef]
- Roulland, E.; Bertounesque, E.; Huel, C.; Monneret, C. Synthesis of picropodophyllin homolactone. Tetrahedron Lett. 2000, 41, 6769–6773. [Google Scholar] [CrossRef]
- Adler, E.; Hernestam, S.; Boss, E.; Caglieris, A. Estimation of Phenolic Hydroxyl Groups in Lignin. I. Periodate Oxidation of Guaiacol Compounds. Acta Chem. Scand. 1955, 9, 319–334. [Google Scholar] [CrossRef]
- LaLonde, R.T.; Ramdayal, F.D.; Sarko, A.; Yanai, K.; Zhang, M. Modes of Methyleneoxy Bridging and Their Effect on Tetrahydronaphthalene Lignan Cytotoxicity. J. Med. Chem. 2003, 46, 1180–1190. [Google Scholar] [CrossRef]
- Ayres, D.C.; Ritchie, T.J. Lignans and related phenols. Part 18. The synthesis of quinones from podophyllotoxin and its analogues. J. Chem. Soc. Perkin Trans. 1 1988, 2573–2578. [Google Scholar] [CrossRef]
- Zhi, X.; Yu, X.; Yang, C.; Ding, G.; Chen, H.; Xu, H. Synthesis of 4β-acyloxypodophyllotoxin analogs modified in the C and E rings as insecticidal agents against Mythimna separata Walker. Bioorg. Med. Chem. Lett. 2014, 24, 765–772. [Google Scholar] [CrossRef]
- Swigor, J.E.; Haynes, U.J. Synthesis of 4-o-(4,6-ethylidene-α-D-glucopyranosyl)- 4′-demethyl-3′-O-[14C]methyl-4-epipodophyllotoxin. J. Label. Compd. Radiopharm. 1990, 28, 137–141. [Google Scholar] [CrossRef]
- Nasveschuk, C.; Rovis, T. A Rapid Total Synthesis of (±)-Sylvone. Synlett 2008, 126–128. [Google Scholar] [CrossRef]
- Vitale, M.; Prestat, G.; Lopes, D.; Madec, D.; Kammerer, C.; Poli, G.; Girnita, L. New Picropodophyllin Analogs via Palladium-Catalyzed Allylic Alkylation−Hiyama Cross-Coupling Sequences. J. Org. Chem. 2008, 73, 5795–5805. [Google Scholar] [CrossRef] [PubMed]
- Stadler, D.; Bach, T. Concise Stereoselective Synthesis of (−)-Podophyllotoxin by an Intermolecular Iron(III)-Catalyzed Friedel-Crafts Alkylation. Angew. Chem. Int. Ed. 2008, 47, 7557–7559. [Google Scholar] [CrossRef] [PubMed]
- Mingoia, F.; Vitale, M.; Madec, D.; Prestat, G.; Poli, G. Pseudo-domino palladium-catalyzed allylic alkylation/Mizoroki–Heck coupling reaction: a key sequence toward (±)-podophyllotoxin. Tetrahedron Lett. 2008, 49, 760–763. [Google Scholar] [CrossRef]
- Pullin, R.D.C.; Sellars, J.D.; Steel, P.G. Silenes in organic synthesis: a concise synthesis of (±)-epi-picropodophyllin. Org. Biomol. Chem. 2007, 5, 3201–3206. [Google Scholar] [CrossRef] [PubMed]
- Akindele, T.; Marsden, S.P.; Cumming, J.G. Stereocontrolled Assembly of Tetrasubstituted Tetrahydrofurans: A Concise Synthesis of Virgatusin. Org. Lett. 2005, 7, 3685–3688. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, K.; Ishiguro, T.; Iitaka, Y.; Koga, K. Asymmetric total synthesis of natural (−)-and unnatural (+)-steganacin: Determination of the absolute configuration of natural antitumor steganacin. Tetrahedron 1984, 40, 1303–1312. [Google Scholar] [CrossRef]
- Fan, J.-C.; Shang, Z.-C.; Liang, J.; Liu, X.-H.; Liu, Y. The oxidation of alcohols to aldehydes and ketones with N -bromosuccinimide in polyethylene glycol: an experimental and theoretical study. J. Phys. Org. Chem. 2008, 21, 945–953. [Google Scholar] [CrossRef]
- Kende, A.S.; Liebeskind, L.S.; Mills, J.E.; Rutledge, P.S.; Curran, D.P. Oxidative aryl-benzyl coupling. A biomimetic entry to podophyllin lignan lactones. J. Am. Chem. Soc. 1977, 99, 7082–7083. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Nakajima, S.; Arimoto, M.; Tanoguchi, M.; Ishida, T.; Inoue, M. Studies on the constituents of the seeds of Hernandia ovigera L. IV. Syntheses of β-peltatin-A and -B methyl ethers from desoxypodophyllotoxin. Chem. Pharm. Bull. (Tokyo) 1984, 32, 1754–1760. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Arimoto, M.; Nakajima, S.; Tanoguchi, M.; Fukada, Y. Studies on the constituents of the seeds of Hernandia ovigera L. V Syntheses of epipodophyllotoxin and podophyllotoxin from desoxypodophyllotoxin. Chem. Pharm. Bull. (Tokyo) 1986, 34, 2056–2060. [Google Scholar] [CrossRef]
- Ghosal, S.; Banerjee, S. Synthesis of retrochinensin; a new naturally occurring 4-aryl-2,3-naphthalide lignan. J. Chem. Soc. Chem. Commun. 1979, 0, 165–166. [Google Scholar] [CrossRef]
- Banerji, J.; Das, B.; Chatterjee, A.; Shoolery, J.N. Gadain, a lignan from jatropha gossypifolia. Phytochemistry 1984, 23, 2323–2327. [Google Scholar] [CrossRef]
- Mincione, E.; Sanetti, A.; Bernini, R.; Felici, M.; Bovicelli, P. Selective oxidation of lignan compounds by dimethyldioxirane. Diastereoselective opening of asarinin furo-furan skeleton. Tetrahedron Lett. 1998, 39, 8699–8702. [Google Scholar] [CrossRef]
- MacLean, H.; Murakami, K. Lignans of western red cedar (Thuja plicata Donn) IV. Thujaplicutin and Thujaplicutin Methyl Ether. Can. J. Chem. 1966, 44, 1541–1545. [Google Scholar] [CrossRef]
- MacLean, H.; Macdonald, B.F. Lignans of western red cedar (Thuja plicata Donn). VII. Dihydroxythujaplicatin. Can. J. Chem. 1967, 45, 739–740. [Google Scholar] [CrossRef]
- Leopold, B.; Malmström, I.-L.; Holtermann, H.; Sörensen, J.S.; Sörensen, N.A. Nitrobenzene Oxidation of Compounds of the Lignan Type. Acta Chem. Scand. 1951, 5, 936–940. [Google Scholar] [CrossRef]
- Wada, K.; Munakata, K. (−) Parabenzlactone, a new piperolignanolide isolated from nakai. Tetrahedron Lett. 1970, 11, 2017–2019. [Google Scholar] [CrossRef]
- Yamauchi, S.; Sugahara, T.; Nakashima, Y.; Okada, A.; Akiyama, K.; Kishida, T.; Maruyama, M.; Masuda, T. Radical and Superoxide Scavenging Activities of Matairesinol and Oxidized Matairesinol. Biosci. Biotechnol. Biochem. 2006, 70, 1934–1940. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, S.; Hayashi, Y.; Nakashima, Y.; Kirikihira, T.; Yamada, K.; Masuda, T. Effect of benzylic oxygen on the antioxidant activity of phenolic lignans. J. Nat. Prod. 2005, 68, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Robin, J.P. A new route to the bis-benzocyclooctadiene lignan skeleton: Total syntheses of (±) picrostegane, (±) isopicrostegane and (±) isostegane. Tetrahedron Lett. 1978, 19, 3613–3616. [Google Scholar] [CrossRef]
- Hajra, S.; Garai, S.; Hazra, S. Catalytic Enantioselective Synthesis of (−)-Podophyllotoxin. Org. Lett. 2017, 19, 6530–6533. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Cong, X.-W.; Yang, G.-Z.; Wang, Y.-W.; Peng, Y. Divergent Asymmetric Syntheses of Podophyllotoxin and Related Family Members via Stereoselective Reductive Ni-Catalysis. Org. Lett. 2018, 20, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Ogihara, Y. Studies on the constituents of enkianthus nudipes. V. A new lignan xyloside from the stems. Chem. Pharm. Bull. (Tokyo) 1976, 24, 2102–2105. [Google Scholar] [CrossRef]
- Brusentsev, Y.; Sandberg, T.; Hotokka, M.; Sjöholm, R.; Eklund, P. Synthesis and structural analysis of sterically hindered chiral 1,4-diol ligands derived from the lignan hydroxymatairesinol. Tetrahedron Lett. 2013, 54, 1112–1115. [Google Scholar] [CrossRef]
- Brusentsev, Y.; Hänninen, M.; Eklund, P. Synthesis of Sterically Hindered Chiral 1,4-Diols from Different Lignan-Based Backbones. Synlett 2013, 24, 2423–2426. [Google Scholar] [CrossRef]
- Markus, H.; Plomp, A.J.; Sandberg, T.; Nieminen, V.; Bitter, J.H.; Murzin, D.Y. Dehydrogenation of hydroxymatairesinol to oxomatairesinol over carbon nanofibre-supported palladium catalysts. J. Mol. Catal. A Chem. 2007, 274, 42–49. [Google Scholar] [CrossRef]
- Simakova, O.A.; Murzina, E.V.; Mäki-Arvela, P.; Leino, A.-R.; Campo, B.C.; Kordás, K.; Willför, S.M.; Salmi, T.; Murzin, D.Y. Oxidative dehydrogenation of a biomass derived lignan – Hydroxymatairesinol over heterogeneous gold catalysts. J. Catal. 2011, 282, 54–64. [Google Scholar] [CrossRef]
- Simakova, O.A.; Smolentseva, E.; Estrada, M.; Murzina, E.V.; Beloshapkin, S.; Willför, S.M.; Simakov, A.V.; Murzin, D.Y. From woody biomass extractives to health-promoting substances: Selective oxidation of the lignan hydroxymatairesinol to oxomatairesinol over Au, Pd, and Au–Pd heterogeneous catalysts. J. Catal. 2012, 291, 95–103. [Google Scholar] [CrossRef]
- Simakova, O.A.; Murzina, E.V.; Leino, A.-R.R.; Mäki-Arvela, P.; Willför, S.; Murzin, D.Y. Gold Catalysts for Selective Aerobic Oxidation of the Lignan Hydroxymatairesinol to Oxomatairesinol: Catalyst Deactivation and Regeneration. Catal. Letters 2012, 142, 1011–1019. [Google Scholar] [CrossRef]
- Prestianni, A.; Ferrante, F.; Simakova, O.A.; Duca, D.; Murzin, D.Y. Oxygen-Assisted Hydroxymatairesinol Dehydrogenation: A Selective Secondary-Alcohol Oxidation over a Gold Catalyst. Chem. Eur. J. 2013, 19, 4577–4585. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Simakova, O.A.; Murzina, E.V.; Willför, S.M.; Prosvirin, I.; Simakov, A.; Murzin, D.Y. Gold particle size effect in biomass-derived lignan hydroxymatairesinol oxidation over Au/Al2O3 catalysts. Appl. Catal. A Gen. 2015, 504, 248–255. [Google Scholar] [CrossRef]
- Khamlach, K.; Dhal, R.; Brown, E. Total syntheses of (−)-trachelogenin, (−)-nortrachelogenin and (+)-wikstromol. Tetrahedron Lett. 1989, 30, 2221–2224. [Google Scholar] [CrossRef]
- Mäkelä, T.H.; Kaltia, S.A.; Wähälä, K.T.; Hase, T.A. α,β-Dibenzyl-γ-butyrolactone lignan alcohols: Total synthesis of (±)-7′-hydroxyenterolactone, (±)-7′-hydroxymatairesinol and (±)-8-hydroxyenterolactone. Steroids 2001, 66, 777–784. [Google Scholar] [CrossRef]
- Chen, B.-C.; Zhou, P.; Davis, F.A.; Ciganek, E. α-Hydroxylation of Enolates and Silyl Enol Ethers. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 1–356. ISBN 9780471264187. [Google Scholar]
- Moritani, Y.; Fukushima, C.; Ukita, T.; Miyagishima, T.; Ohmizu, H.; Iwasaki, T. Stereoselective syntheses of cis- and trans-isomers of α-hydroxy-α,β-dibenzyl-γ-butyrolactone lignans: New syntheses of (±)-trachelogenin and (±)-guayadequiol. J. Org. Chem. 1996, 61, 6922–6930. [Google Scholar] [CrossRef] [PubMed]
- Moritani, Y.; Ukita, T.; Hiramatsu, H.; Okamura, K.; Ohmizu, H.; Iwasaki, T. A highly stereoselective synthesis of 3-hydroxy-1-aryltetralin lignans based on the stereoselective hydroxylation of α,β-dibenzyl-γ-butyro-lactones: the first synthesis of (±)-cycloolivil. J. Chem. Soc., Perkin Trans. 1 1996, 0, 2747–2753. [Google Scholar] [CrossRef]
- Kramer, B.; Fröhlich, R.; Waldvogel, S.R. Oxidative Coupling Reactions Mediated by MoCl5 Leading to 2,2′-Cyclolignans: The Specific Role of HCl. Eur. J. Org. Chem. 2003, 18, 3549–3554. [Google Scholar] [CrossRef]
- Kramer, B.; Averhoff, A.; Waldvogel, S.R. Highly selective formation of eight-membered-ring systems by oxidative cyclization with molybdenum pentachloride—An environmentally friendly and inexpensive access to 2,2′-cyclolignans. Angew. Chem. Int. Ed. 2002, 41, 2981–2982. [Google Scholar] [CrossRef]
- Damon, R.E.; Schlessinger, R.H.; Blount, J.F. A short synthesis of (.+-.)-isostegane. J. Org. Chem. 1976, 41, 3772–3773. [Google Scholar] [CrossRef]
- Landais, Y.; Lebrun, A.; Robin, J.P. Ruthenium(IV) tetrakis(trifluoroacetate), a new oxidizing agent. II. A new access to schizandrins skeleton using biaryl oxidative coupling of cis-substituted butanolides. Tetrahedron Lett. 1986, 27, 5377–5380. [Google Scholar] [CrossRef]
- Landais, Y.; Robin, J.-P. Le tétrakis (trifluoroacétate) de ruthénium(IV), nouveau catalyseur a température ambiante du couplage biarylique oxydant non phénolique -première synthèse totale biomimétique du néoisostégane-. Tetrahedron Lett. 1986, 27, 1785–1788. [Google Scholar] [CrossRef]
- Robin, J.P.; Landais, Y. Ruthenium(IV) dioxide in fluoro acid medium. An efficient biaryl phenol coupling process, exemplified with a biomimetic access to the skeleton of steganacin from presteganes. J. Org. Chem. 1988, 53, 224–226. [Google Scholar] [CrossRef]
- Landais, Y.; Robin, J.-P.; Lebrun, A. Ruthenium dioxide in fluoro acid medium: I. A new agent in the biaryl oxidative coupling. Application to the synthesis of non phenolic bisbenzocyclooctadiene lignan lactones. Tetrahedron 1991, 47, 3787–3804. [Google Scholar] [CrossRef]
- Robin, J.-P.; Landais, Y. Ruthenium dioxide in fluoro acid medium: II. Application to the formation of steganes skeleton by oxidative phenolic coupling. Tetrahedron 1992, 48, 819–830. [Google Scholar] [CrossRef]
- Dhal, R.; Landais, Y.; Lebrun, A.; Lenain, V.; Robin, J.-P. Ruthenium dioxide in fluoro acid medium V. Application to the non phenolic oxidative coupling of diarylbutanes. Conformational studies of cis and trans deoxyschizandrins. Tetrahedron 1994, 50, 1153–1164. [Google Scholar] [CrossRef]
- Pettit, G.R.; Meng, Y.; Gearing, R.P.; Herald, D.L.; Pettit, R.K.; Doubek, D.L.; Chapuis, J.-C.; Tackett, L.P. Antineoplastic Agents. 522. Hernandia peltata (Malaysia) and Hernandia nymphaeifolia (Republic of Maldives). J. Nat. Prod. 2004, 67, 214–220. [Google Scholar] [CrossRef]
- Landais, Y.; Lebrun, A.; Lenain, V.; Robin, J.-P. Synthesis of diarylbutanes from cordigerines and reinvestigation of their oxidative couplings in deoxyschizandrins.—An unusual formation of phenyltetralin lignans -. Tetrahedron Lett. 1987, 28, 5161–5164. [Google Scholar] [CrossRef]
- Cambie, R.; Craw, P.; Rutledge, P.; Woodgate, P. Oxidative Coupling of Lignans. III. Non-Phenolic Oxidative Coupling of Deoxypodorhizon and Related Compounds. Aust. J. Chem. 1988, 41, 897–918. [Google Scholar] [CrossRef]
- Burden, J.; Cambie, R.; Craw, P.; Rutledge, P.; Woodgate, P. Oxidative Coupling of Lignans. IV. Monophenolic Oxidative Coupling. Aust. J. Chem. 1988, 41, 919–933. [Google Scholar] [CrossRef]
- Planchenault, D.; Dhal, R.; Robin, J.-P. New agents of biaryl oxidative coupling in fluoro acid medium. VI. Application to the synthesis of phenolic bisbenzocyclooctadiene lignans. Tetrahedron 1995, 51, 1395–1404. [Google Scholar] [CrossRef]
- Ward, R.S.; Hughes, D.D. Oxidative cyclisation of cis- and trans-2,3-dibenzylbutyrolactones using ruthenium tetra(trifluoroacetate). Tetrahedron 2001, 57, 4015–4022. [Google Scholar] [CrossRef]
- Planchenault, D.; Dhal, R.; Robin, J.-P. Synthesis of non-phenolic bisbenzocyclooctadiene lignan lactones and aporphinic alkaloids, by oxidative coupling with new agents in fluoro acid medium. IV. Tetrahedron 1993, 49, 5823–5830. [Google Scholar] [CrossRef]
- Saladino, R.; Fiani, C.; Belfiore, M.C.; Gualandi, G.; Penna, S.; Mosesso, P. Methyltrioxorhenium catalysed synthesis of highly oxidised aryltetralin lignans with anti-topoisomerase II and apoptogenic activities. Bioorganic Med. Chem. 2005, 13, 5949–5960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saladino, R.; Fiani, C.; Crestini, C.; Argyropoulos, D.S.; Marini, S.; Coletta, M. An Efficient and Stereoselective Dearylation of Asarinin and Sesamin Tetrahydrofurofuran Lignans to Acuminatolide by Methyltrioxorhenium/H2 O2 and UHP Systems. J. Nat. Prod. 2007, 70, 39–42. [Google Scholar] [CrossRef]
- Bernini, R.; Gualandi, G.; Crestini, C.; Barontini, M.; Belfiore, M.C.; Willför, S.; Eklund, P.; Saladino, R. A novel and efficient synthesis of highly oxidized lignans by a methyltrioxorhenium/hydrogen peroxide catalytic system. Studies on their apoptogenic and antioxidant activity. Bioorg. Med. Chem. 2009, 17, 5676–5682. [Google Scholar] [CrossRef] [PubMed]
- Nishibe, S.; Chiba, M.; Sakushima, A.; Hisada, S.; Yamanouchi, S.; Takido, M.; Sankawa, U.; Sakakibara, A. Introduction of an alcoholic hydroxyl group into 2,3-dibenzylbutyrolactone lignans with oxidizing agents and carbon-13 nuclear magnetic resonance spectra of the oxidation products. Chem. Pharm. Bull. (Tokyo) 1980, 28, 850–860. [Google Scholar] [CrossRef]
- Pohmakotr, M.; Pinsa, A.; Mophuang, T.; Tuchinda, P.; Prabpai, S.; Kongsaeree, P.; Reutrakul, V. General strategy for stereoselective synthesis of 1-substituted Exo,Endo-2,6-diaryl-3,7-dioxabicyclo[3.3.0]octanes: Total synthesis of (±)-gmelinol. J. Org. Chem. 2006, 71, 386–389. [Google Scholar] [CrossRef]
- Takemoto, M.; Aoshima, Y.; Stoynov, N.; Kutney, J.P. Establishment of Camellia sinensis cell culture with high peroxidase activity and oxidative coupling reaction of dibenzylbutanolides. Tetrahedron Lett. 2002, 43, 6915–6917. [Google Scholar] [CrossRef]
- Takemoto, M.; Fukuyo, A.; Aoshima, Y.; Tanaka, K. Synthesis of Lyoniresinol with Combined Utilization of Synthetic Chemistry and Biotechnological Methods. Chem. Pharm. Bull. (Tokyo) 2006, 54, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Kutney, J.P.; Xinyao, D.; Naidu, R.; Stoynov, N.M.; Takemoto, M. Biotransformation of Dibenzylbutanolides by Peroxidase Enzymes. Routes to the Podophyllotoxin Family. Heterocycles 1996, 42, 479–484. [Google Scholar] [CrossRef]
- Yue, F.; Lu, F.; Ralph, S.; Ralph, J. Identification of 4–O–5-Units in Softwood Lignins via Definitive Lignin Models and NMR. Biomacromolecules 2016, 17, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Moinuddin, S.G.A.; Youn, B.; Bedgar, D.L.; Costa, M.A.; Helms, G.L.; Kang, C.; Davin, L.B.; Lewis, N.G. Secoisolariciresinol dehydrogenase: mode of catalysis and stereospecificity of hydride transfer in Podophyllum peltatum. Org. Biomol. Chem. 2006, 4, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Murata, J.; Ono, E.; Yoroizuka, S.; Toyonaga, H.; Shiraishi, A.; Mori, S.; Tera, M.; Azuma, T.; Nagano, A.J.; Nakayasu, M.; Mizutani, M.; Wakasugi, T.; Yamamoto, M.P.; Horikawa, M. Oxidative rearrangement of (+)-sesamin by CYP92B14 co-generates twin dietary lignans in sesame. Nat. Commun. 2017, 8, 2155. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, N.P.; Julsing, M.K.; Koulman, A.; Clarkson, C.; Woerdenbag, H.J.; Ionkova, I.; Bos, R.; Jaroszewski, J.W.; Kayser, O.; Quax, W.J. Bioconversion of deoxypodophyllotoxin into epipodophyllotoxin in E. coli using human cytochrome P450 3A4. J. Biotechnol. 2006, 126, 383–393. [Google Scholar] [CrossRef]
- Krauss, A.S.; Taylor, W.C. Intramolecular Oxidative Coupling of Aromatic Compounds. III. Monophenolic Substrates. Aust. J. Chem. 1992, 45, 925–933. [Google Scholar] [CrossRef]
- Fernandes, J.B.; Fraga, R.L.; Capelato, M.D.; Vierira, P.C.; Yoshida, M.; Kato, M.J. Synthesis of Dibenzocyclooctadienes by Anodic Oxidation. Synth. Commun. 1991, 21, 1331–1336. [Google Scholar] [CrossRef]
- Kawamura, F.; Miyachi, M.; Kawai, S.; Ohashi, H. Photodiscoloration of western hemlock (Tsuga heterophylla) sapwood III Early stage of photodiscoloration reaction with lignans. J. Wood Sci. 1998, 44, 47–55. [Google Scholar] [CrossRef]
- Litescu, S.C.; Eremia, S.A.V.; Tache, A.; Vasilescu, I.; Radu, G.-L. The Use of Oxygen Radical Absorbance Capacity (ORAC) and Trolox Equivalent Antioxidant Capacity (TEAC) Assays in the Assessment of Beverages’ Antioxidant Properties. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 245–251. ISBN 978-0-12-404738-9. [Google Scholar]
- Golubev, V.A.; Rozantsev, E.G.; Neiman, M.B. Some reactions of free iminoxyl radicals with unpaired electron participation. Izv. Akad. Nauk SSSR Ser. Khim. 1965, 11, 1927–1936. [Google Scholar] [CrossRef]
- Merbouh, N.; Bobbitt, J.M.; Brückner, C. Preparation of Tetramethylpiperidine-1-oxoammonium Salts And Their Use As Oxidants In Organic Chemistry. A Review. Org. Prep. Proced. Int. 2004, 36, 1–31. [Google Scholar] [CrossRef]
- Tojo, G.; Fernández, M.I. TEMPO-Mediated Oxidations. In Oxidation of Primary Alcohols to Carboxylic Acids; Springer: New York, NY, USA, 2007; pp. 79–103. [Google Scholar]
- Nandanwar, R.A.; Chaudhari, A.R.; Ekhe, J.D. Nitrobenzene Oxidation for Isolation of Value Added Products from Industrial Waste Lignin. J. Chem. Biol. Phys. Sci. 2016, 6, 501–513. [Google Scholar]
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Huis, R.; Morreel, K.; Fliniaux, O.; Lucau-Danila, A.; Fénart, S.; Grec, S.; Neutelings, G.; Chabbert, B.; Mesnard, F.; Boerjan, W.; Hawkins, S. Natural hypolignification is associated with extensive oligolignol accumulation in flax stems. Plant Physiol. 2012, 158, 1893–1915. [Google Scholar] [CrossRef] [PubMed]
- Glazunova, O.A.; Trushkin, N.A.; Moiseenko, K.V.; Filimonov, I.S.; Fedorova, T.V. Catalytic Efficiency of Basidiomycete Laccases: Redox Potential versus Substrate-Binding Pocket Structure. Catalysts 2018, 8, 152. [Google Scholar] [CrossRef]
- Moya, R.; Saastamoinen, P.; Hernández, M.; Suurnäkki, A.; Arias, E.; Mattinen, M.-L. Reactivity of bacterial and fungal laccases with lignin under alkaline conditions. Bioresour. Technol. 2011, 102, 10006–10012. [Google Scholar] [CrossRef] [PubMed]
- Buchert, J.; Mustranta, A.; Tamminen, T.; Spetz, P.; Holmbom, B. Modification of Spruce Lignans with Trametes hirsuta Laccase. Holzforschung 2002, 56, 579–584. [Google Scholar] [CrossRef]
- Maijala, P.; Mattinen, M.-L.; Nousiainen, P.; Kontro, J.; Asikkala, J.; Sipilä, J.; Viikari, L. Action of fungal laccases on lignin model compounds in organic solvents. J. Mol. Catal. B Enzym. 2012, 76, 59–67. [Google Scholar] [CrossRef]
- Mattinen, M.-L.; Maijala, P.; Nousiainen, P.; Smeds, A.; Kontro, J.; Sipilä, J.; Tamminen, T.; Willför, S.; Viikari, L. Oxidation of lignans and lignin model compounds by laccase in aqueous solvent systems. J. Mol. Catal. B Enzym. 2011, 72, 122–129. [Google Scholar] [CrossRef]
- Mattinen, M.-L.; Struijs, K.; Suortti, T.; Mattila, I.; Kruus, K.; Willför, S.; Tamminen, T.; Vincken, J.-P. Modification of Lignans by Trameter Hirsuta Laccase. BioResources 2009, 4, 482–496. [Google Scholar] [CrossRef]
- Frív, I.; Siverio, J.M.; González, C.; Trujillo, J.M.; Pérez, J.A. Purification of a new peroxidase catalysing the formation of lignan-type compounds. Biochem. J. 1991, 273 (Pt 1), 109–113. [Google Scholar] [CrossRef] [Green Version]
- Hazra, S.; Chattopadhyay, S. An overview of lignans with special reference to podophyllotoxin, a cytotoxic lignan. Chem. Biol. Lett. 2016, 3, 1–8. [Google Scholar]
- Nakatsubo, T.; Mizutani, M.; Suzuki, S.; Hattori, T.; Umezawa, T. Characterization of Arabidopsis thaliana Pinoresinol Reductase, a New Type of Enzyme Involved in Lignan Biosynthesis. J. Biol. Chem. 2008, 283, 15550–15557. [Google Scholar] [CrossRef]
- Xia, Z.Q.; Costa, M.A.; Pelissier, H.C.; Davin, L.B.; Lewis, N.G. Secoisolariciresinol dehydrogenase purification, cloning, and functional expression. Implications for human health protection. J. Biol. Chem. 2001, 276, 12614–12623. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, W.; Bos, J.A.; Boeke, G.M.; Woerdenbag, H.J.; Pras, N. The Large-Scale Isolation of Deoxypodophyllotoxin from Rhizomes of Anthriscus sylvestris Followed by Its Bioconversion into 5-Methoxypodophyllotoxin β- d -Glucoside by Cell Cultures of Linum flavum. J. Nat. Prod. 1997, 60, 401–403. [Google Scholar] [CrossRef]
- Oliveira, T.L.S.; de Siqueira Leite, K.C.; de Macêdo, I.Y.L.; de Morais, S.R.; Costa, E.A.; de Paula, J.R.; de Souza Gil, E. Electrochemical Behavior and Antioxidant Activity of Hibalactone. Int. J. Electrochem. Sci. 2017, 12, 7596–7964. [Google Scholar] [CrossRef]
- Silva, D.H.S.; Pereira, F.C.; Zanoni, M.V.B.; Yoshida, M. Lipophyllic antioxidants from Iryanthera juruensis fruits. Phytochemistry 2001, 57, 437–442. [Google Scholar] [CrossRef]
- Yang, X.; Gao, M.; Hu, H.; Zhang, H. Electrochemical detection of honokiol and magnolol in traditional Chinese medicines using acetylene black nanoparticle-modified Electrode. Phytochem. Anal. 2011, 22, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Amine, A.; Brett, C.M.A.; Oliveira-Brett, A.M. Virgin olive oil ortho-phenols—electroanalytical quantification. Talanta 2013, 105, 179–186. [Google Scholar] [CrossRef]
- Natale, A.; Nardiello, D.; Palermo, C.; Muscarella, M.; Quinto, M.; Centonze, D. Development of an analytical method for the determination of polyphenolic compounds in vegetable origin samples by liquid chromatography and pulsed amperometric detection at a glassy carbon electrode. J. Chromatogr. A 2015, 1420, 66–73. [Google Scholar] [CrossRef]
- Fernández, E.; Vidal, L.; Canals, A. Rapid determination of hydrophilic phenols in olive oil by vortex-assisted reversed-phase dispersive liquid-liquid microextraction and screen-printed carbon electrodes. Talanta 2018, 181, 44–51. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Voltammetric e-tongue for the quantification of total polyphenol content in olive oils. Food Res. Int. 2013, 54, 2075–2082. [Google Scholar] [CrossRef]
- Przygodzka, M.; Zielińska, D.; Ciesarová, Z.; Kukurová, K.; Zieliński, H. Comparison of methods for evaluation of the antioxidant capacity and phenolic compounds in common spices. LWT - Food Sci. Technol. 2014, 58, 321–326. [Google Scholar] [CrossRef]
- Rivas Romero, M.P.; Estévez Brito, R.; Rodríguez Mellado, J.M.; González-Rodríguez, J.; Ruiz Montoya, M.; Rodríguez-Amaro, R. Exploring the relation between composition of extracts of healthy foods and their antioxidant capacities determined by electrochemical and spectrophotometrical methods. LWT 2018, 95, 157–166. [Google Scholar] [CrossRef]
- Radi, A.-E.; Abd-Elghany, N.; Wahdan, T. Electrochemical study of the antineoplastic agent etoposide at carbon paste electrode and its determination in spiked human serum by differential pulse voltammetry. Chem. Pharm. Bull. (Tokyo) 2007, 55, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.M.; Khamis, E.F.; El-Kimary, E.I.; Barary, M.A. Development of a differential pulse voltammetric method for the determination of Silymarin/Vitamin E acetate mixture in pharmaceuticals. Talanta 2008, 74, 773–778. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, H.S.; Ghoneim, M.M. Stripping voltammetric determination of silymarin in formulations and human blood utilizing bare and modified carbon paste electrodes. Talanta 2011, 84, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Mpanza, T.; Sabela, M.I.; Mathenjwa, S.S.; Kanchi, S.; Bisetty, K. Electrochemical Determination of Capsaicin and Silymarin Using a Glassy Carbon Electrode Modified by Gold Nanoparticle Decorated Multiwalled Carbon Nanotubes. Anal. Lett. 2014, 47, 2813–2828. [Google Scholar] [CrossRef]
- Gažák, R.; Svobodová, A.; Psotová, J.; Sedmera, P.; Přikrylová, V.; Walterová, D.; Křen, V. Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorganic Med. Chem. 2004, 12, 5677–5687. [Google Scholar] [CrossRef]
- Kosina, P.; Kren, V.; Gebhardt, R.; Grambal, F.; Ulrichova, J.; Walterova, D. Antioxidant properties of silybin glycosides. Phytother Res 2002, 16, 33–39. [Google Scholar] [CrossRef]




















































| Non-Metal Ox. | Oxidative Transformation | Yields | Ref. | |
| DDQ | Benzylic hydride abstraction and benzylic ring closures, Ar-Ar-coupling to dibenzocyclooctadiene, nucleophilic attack, alcohol oxidation, aromatization | ~10–40% | [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] | |
| mCPBA | Epoxidation, Baeyer-Villiger oxidation, oxidation of ethoxy-THF-lignans to lactones | ~70–90% | [29,38,39,40,41,42] | |
| peroxyl radical (ROO.) | Antioxidative radical scavenging to phenoxyl radicals and further radical couplings | No data | [43,44,45,46] | |
| Azo compounds (AAPH, AIBN, ABTS) | Same as above | No data | [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] | |
| DPPH | Same as above, radical 5-5 couplings to dimers and oxidation of benzylic alcohol to ketone | No data | [6,51,64,65,66,67] | |
| TEMPO | Oxidation of benzylic alcohol to ketone | 60% | [68] | |
| BAIB and PIFA | phenolic hydroxyl oxidation to quinone type structures, Ar-Ar-coupling to dibenzocyclooctadienes, nucleophilic attack at ipso- or benzylic position | ~10–80% | [69,70,71,72,73,74] | |
| IBX | Aromatic demethylation, benzylic alcohol oxidation to carbonyl | ~25–95% | [75,76,77,78,79] | |
| Dess-Martin Periodinane (DMP) | Benzylic alcohol oxidation to carbonyl, 9,9′-diol oxidation to lactols | >90% | [80,81,82,83,84,85,86,87,88,89] | |
| NaIO4 | Oxidation of guaiacyl or syringyl groups to demethylated o-quinones, Lemieux–Johnson oxidation | ~50–95% | [90,91,92,93,94,95,96,97,98,99,100] | |
| N-Bromosuccinimide (NBS) | Brominations (arylic or benzylic), benzylic CH2 to ketone, benzylic ring closure and aromatization | 75–90% | [35,101,102,103,104,105,106,107] | |
| Dimethyldioxirane (DMDO) | Oxidative ring opening of furan rings | ~80% | [108] | |
| Nitrobenzene | Oxidative degradation to vanillin and vanillic acid | 80–100% | [109,110,111] | |
| Metal-Mediated Ox. | Oxidative Transformation | Yields | Ref. | |
| Cr-(VI) (CrO3, PCC, PDC) | Oxidation of benzylic alcohols to ketones, primary alcohols into lactones and carboxylic acid | ~60–95% | [87,103,105,112,113,114,115,116,117,118,119,120] | |
| Pd, Au | Oxidation of benzylic alcohols in presence of free phenolic into (mainly) ketone | No data | [121,122,123,124,125,126] | |
| MoOPH | α-Hydroxylation | ~25–95% | [113,127,128,129,130,131] | |
| MoCl5 | Ar-Ar-coupling to dibenzocyclooctadienes | ~50–90% | [132,133] | |
| VoF3; V2O5; Tl2O3; RuO5 | Ar-Ar-coupling to dibenzocyclooctadienes, benzylic ring closure | ~50–100% | [134,135,136,137,138,139,140,141,142,143,144,145,146,147] | |
| MTO (catalyst) | Aromatic demethylation and quinone formation and simultaneous benzylic alcohol oxidation to ketones, benzylic cleavage, benzylic hydroxylation, oxidation of benzylic alcohol to ketone | ~40–100% | [148,149,150] | |
| Pb(OAc)4 | Benzylic acetoxylation | ~30–70% | [151] | |
| CeCl3 | α-Hydroxylation | 71% | [152] | |
| Other Oxidations | Oxidative Transformation | Yields | Ref. | |
| Enzymatic ox. | peroxidase | Benzylic ring closure | 37–99% | [153,154,155] |
| HRP | Radical 4-O-5 coupling | 3.6% | [156] | |
| SDH | Oxidation of diol to lactone | No data | [157] | |
| P450/CPR1 | Arylic hydroxylation and rearrangement | No data | [158] | |
| CYP | Benzylic hydroxylation | 90% | [159] | |
| Electrochemical ox. | Demethylation, quinone formation, and benzylic ring closure | No data | [160] | |
| Ar-Ar-coupling to dibenzocyclooctadiene | >80% | [161] | ||
| Photooxidation | Benzylic: cleavage/alcohol oxidation to ketone/ring closure/nucleophilic attack | No data | [162] | |
| Lignan | Vanillin % | Vanillic Acid % | Conversion % |
|---|---|---|---|
| Pinoresinol | 31 | 9 | 81 |
| Lariciresinol | 63 | 5 | 100 |
| Olivil | 83 | 3 | 100 |
| Matairesinol | 15 | 2 | 100 |
| Conidendrin | 1 | - | - |
| Isoolivil | 3 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Runeberg, P.A.; Brusentsev, Y.; Rendon, S.M.K.; Eklund, P.C. Oxidative Transformations of Lignans. Molecules 2019, 24, 300. https://doi.org/10.3390/molecules24020300
Runeberg PA, Brusentsev Y, Rendon SMK, Eklund PC. Oxidative Transformations of Lignans. Molecules. 2019; 24(2):300. https://doi.org/10.3390/molecules24020300
Chicago/Turabian StyleRuneberg, Patrik A., Yury Brusentsev, Sabine M. K. Rendon, and Patrik C. Eklund. 2019. "Oxidative Transformations of Lignans" Molecules 24, no. 2: 300. https://doi.org/10.3390/molecules24020300
APA StyleRuneberg, P. A., Brusentsev, Y., Rendon, S. M. K., & Eklund, P. C. (2019). Oxidative Transformations of Lignans. Molecules, 24(2), 300. https://doi.org/10.3390/molecules24020300