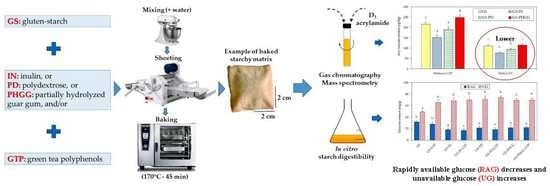
Effect of the Addition of Soluble Dietary Fiber and Green Tea Polyphenols on Acrylamide Formation and In Vitro Starch Digestibility in Baked Starchy Matrices
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Behavior of the Soluble Dietary Fibers (SDFs) Used in Starchy Matrices
2.2. Changes of Water Activity (aw) and Moisture Content of Starchy Matrices
2.3. Textural Characteristics of Doughs before Baking
2.4. Textural Changes of Starchy Matrices after Baking
2.5. Acrylamide Content of Baked Starchy Matrices
2.6. In Vitro Starch Digestibility of Baked Starchy Matrices
3. Discussion
3.1. Textural Characteristics of Starchy Matrices before and after Baking
3.2. Effect of SDF and Green Tea Polyphenols (GTP) on Acrylamide (AA) Reduction in Baked Starchy Matrices
3.3. Effect of SDF and GTP on In Vitro Starch Digestibility in Baked Starchy Matrices
4. Materials and Methods
4.1. Materials and Supplies
4.2. Assessment of Physicochemical Properties of SDF
4.2.1. Water-Holding Capacity (WHC)
4.2.2. Apparent Viscosity
4.3. Samples Preparation
4.4. Mechanical and Physical Methods
4.4.1. Moisture Content of Starchy Matrices
4.4.2. Water Activity (aw) of Starchy Matrices
4.4.3. Textural Changes of Starchy Matrices before Baking
4.4.4. Textural Changes of Starchy Matrices after Baking
4.5. Chemical and Analytical Methods
4.5.1. Determination of AA Content in Baked Starchy Matrices
4.5.2. Assessment of In Vitro Starch Digestibility in Baked Starchy Matrices
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhital, S.; Warren, F.; Butterworth, P.; Ellis, P.; Gidley, M. Mechanisms of starch digestion by α-amylase-structural basis for kinetic properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef]
- Vujić, L.; Vitali Čepo, D.; Vedrina Dragojević, I. Impact of dietetic tea biscuit formulation on starch digestibility and selected nutritional and sensory characteristics. LWT Food Sci. Technol. 2015, 62, 647–653. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protocols 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Contardo, I.; Parada, J.; Leiva, A.; Bouchon, P. The effect of vacuum frying on starch gelatinization and its in vitro digestibility in starch-gluten matrices. Food Chem. 2016, 197, 353–358. [Google Scholar] [CrossRef]
- Djurle, S.; Andersson, A.; Andersson, R. Effects of baking on dietary fibre, with emphasis on β-glucan and resistant starch, in barley breads. J. Cereal Sci. 2018, 79, 449–455. [Google Scholar] [CrossRef]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Sivam, A.; Sun-Waterhouse, D.; Quek, S.; Perera, C. Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Englyst, K.; Englyst, H.; Hudson, G.; Cole, T.; Cummings, J. Rapidly available glucose in foods: An in vitro measurement that reflects the glycemic response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef]
- Englyst, K.; Vinoy, S.; Englyst, H.N.; Lang, V. Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br. J. Nutr. 2003, 89, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.; Wonik, J.; Bindrich, U.; Heinemann, M.; Kohrs, H.; Schneider, I.; Möller, K.; Hahn, A. Glycemic index and microstructure analysis of a newly developed fiber enriched cookie. Food Funct. 2016, 7, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterization, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Brennan, C.; Blake, D.; Ellis, P.; Schofield, J. Effects of guar galactomannan on wheat bread microstructure and on the in vitro and in vivo digestibility of starch in bread. J. Cereal Sci. 1996, 24, 151–160. [Google Scholar] [CrossRef]
- Brennan, C.; Kuri, V.; Tudorica, C. Inulin-enriched pasta: Effects on textural properties and starch degradation. Food Chem. 2004, 86, 189–193. [Google Scholar] [CrossRef]
- Dikeman, C.; Murphy, M.; Fahey, G. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. J. Nutr. 2006, 136, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Fabek, H.; Goff, H. Simulated intestinal hydrolysis of native tapioca starch: Understanding the effect of soluble fibre. Bioactive Carbohydr. Diet. Fibre. 2015, 6, 83–98. [Google Scholar] [CrossRef]
- Peressini, D.; Sensidoni, A. Effect of soluble dietary fibre addition on rheological and breadmaking properties of wheat doughs. J. Cereal Sci. 2009, 49, 190–201. [Google Scholar] [CrossRef]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New horizons for the study of dietary fiber and health: A review. Plant Foods Human Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B. Optimization of textural properties of noodles with soluble fiber, dough mixing time and different water levels. J. Cereal Sci. 2016, 69, 104–110. [Google Scholar] [CrossRef]
- Kawai, K.; Hando, K.; Thuwapanichayanan, R.; Hagura, Y. Effect of stepwise baking on the structure, browning, texture, and in vitro starch digestibility of cookie. LWT Food Sci. Technol. 2016, 66, 384–389. [Google Scholar] [CrossRef]
- Pedreschi, F.; Saavedra, I.; Bunger, A.; Zuñiga, R.; Pedreschi, R.; Chirinos, R.; Mariotti-Celis, M. Tara pod (Caesalpinia spinosa) extract mitigates neo-contaminant formation in Chilean bread preserving their sensory attributes. LWT-Food Sci. Technol. 2018, 95, 116–122. [Google Scholar] [CrossRef]
- Mariotti-Celis, M.; Cortés, P.; Dueik, V.; Bouchon, P.; Pedreschi, F. Application of Vacuum Frying as a Furan and Acrylamide Mitigation Technology in Potato Chips. Food Bioprocess Technol. 2017, 10, 2092–2099. [Google Scholar] [CrossRef]
- Keramat, J.; LeBail, A.; Prost, C.; Jafari, M. Acrylamide in baking products: A review article. Food Bioprocess Technol. 2011, 4, 530–543. [Google Scholar] [CrossRef]
- Mottram, D.; Wedzicha, B.; Dodson, A. Food Chemistry: Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Pedreschi, F.; Mariotti, M.; Granby, K.; Risum, J. Acrylamide reduction in potato chips by using commercial asparaginase in combination with conventional blanching. LWT Food Sci. Technol. 2011, 44, 1473–1476. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, K.; Du, Y.; Kong, R.; Lo, C.; Chu, I.; Wang, M. Activities of hydrocolloids as inhibitors of acrylamide formation in model systems and fried potato strips. Food Chem. 2010, 121, 424–428. [Google Scholar] [CrossRef]
- Passos, C.; Ferreira, S.; Serôdio, A.; Basil, E.; Marková, L.; Kukurová, K.; Coimbra, M. Pectic polysaccharides as an acrylamide mitigation strategy-Competition between reducing sugars and sugar acids. Food Hydrocoll. 2018, 81, 113–119. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.; Gutiérrez-Uribe, J.; Serna-Saldívar, S. Bound phenolics in foods: A review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Peng, S.; Zhang, G. Effect of green tea catechins on the postprandial glycemic response to starches differing in amylose content. J. Agric. Food Chem. 2011, 59, 4582–4588. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.; Roberts, T. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Bora, F.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer-Musa, M.; Griffith, A.; Michels, A.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and a-glucosidase activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, P.; Chen, F.; Yuan, Y.; Zhu, Y.; Yan, H.; Hu, X. Role of plant polyphenols in acrylamide formation and elimination. Food Chem. 2015, 186, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Goh, R.; Gao, J.; Ananingsih, V.; Ranawana, V.; Henry, C.; Zhou, W. Green tea catechins reduced the glycaemic potential of bread: An in vitro digestibility study. Food Chem. 2015, 180, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y. Effect of natural antioxidants on kinetic behavior of acrylamide formation and elimination in low-moisture asparagine–glucose model system. J. Food Eng. 2008, 85, 105–115. [Google Scholar] [CrossRef]
- Fu, Z.; Yoo, M.; Zhou, W.; Zhang, L.; Chen, Y.; Lu, J. Effect of (−)-epigallocatechin gallate (EGCG) extracted from green tea in reducing the formation of acrylamide during the bread baking process. Food Chem. 2018, 242, 162–168. [Google Scholar] [CrossRef]
- Dikeman, C.; Fahey, G. Viscosity as related to dietary fiber: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 649–663. [Google Scholar] [CrossRef]
- Rosell, C.; Santos, E.; Collar, C. Physico-chemical properties of commercial fibers from different sources: A comparative approach. Food Res. Inter. 2009, 42, 176–184. [Google Scholar] [CrossRef]
- Mancebo, C.; Rodríguez, P.; Martínez, M.; Gómez, M. Effect of the addition of soluble (nutriose, inulin and polydextrose) and insoluble (bamboo, potato and pea) fibers on the quality of sugar-snap cookies. Inter. J. Food Sci. Technol. 2018, 53, 129–136. [Google Scholar] [CrossRef]
- Collar, C.; Santos, E.; Rosell, C. Assessment of the rheological profile of fibre-enriched bread doughs by response surface methodology. J. Food Eng. 2007, 78, 820–826. [Google Scholar] [CrossRef]
- Kaur, S.; Das, M. Study on the effect of concentration and temperature on rheological properties of whole barley flour suspension by using Mitschka method. J. Texture Stud. 2014, 45, 164–171. [Google Scholar] [CrossRef]
- Rodríguez-García, J.; Laguna, L.; Puig, A.; Salvador, A.; Hernando, I. Effect of fat replacement by inulin on textural and structural properties of short dough biscuits. Food Bioprocess Technol. 2013, 6, 2739–2750. [Google Scholar] [CrossRef]
- Labuza, T.; Mcnally, L.; Gallagher, D.; Hawkes, J.; Hurtado, F. Stability of intermediate moisture foods. J. Food Sci. 1972, 37, 154–159. [Google Scholar] [CrossRef]
- Mathlouthi, M. Water content, water activity, water structure and the stability of foodstuffs. Food Control 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Li, Q.; Liu, R.; Wu, T.; Wang, M.; Zhang, M. Soluble dietary fiber fractions in wheat bran and their interactions with wheat gluten have impacts on dough properties. J. Agric. Food Chem. 2016, 64, 8735–8744. [Google Scholar] [CrossRef]
- Wang, J.; Rosell, C.; Barber, C. Effect of the addition of different fibers on wheat dough performance and bread quality. Food Chem. 2002, 79, 221–226. [Google Scholar] [CrossRef]
- Gao, Y.; Janes, M.; Chaiya, B.; Brennan, M.; Brennan, C.; Prinyawiwatkul, W. Gluten-free bakery and pasta products: Prevalence and quality improvement. Int. J. Food Sci. Technol. 2018, 53, 19–32. [Google Scholar] [CrossRef]
- Dueik, V.; Sobukola, O.; Bouchon, P. Development of low-fat gluten and starch fried matrices with high fiber content. LWT Food Sci. Technol. 2014, 59, 6–11. [Google Scholar] [CrossRef]
- Ronda, F.; Pérez-Quirce, S.; Angioloni, A.; Collar, C. Impact of viscous dietary fibers on the viscoelastic behavior of gluten-free formulated rice doughs: A fundamental and empirical rheological approach. Food Hydrocoll. 2013, 32, 252–262. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W. Stability of tea catechins in the breadmaking process. J. Agric. Food Chem. 2004, 52, 8224–8229. [Google Scholar] [CrossRef]
- Li, Q.; Liu, R.; Wu, T.; Zhang, M. Interactions between soluble dietary fibers and wheat gluten in dough studied by confocal laser scanning microscopy. Food Res. Int. 2017, 95, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Mohammed, I.; Linter, B.; Allen, R.; Charalambides, M. Mechanical and microstructural changes of cheese cracker dough during baking. LWT-Food Sci. Technol. 2017, 86, 148–158. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.; Ayala-Zavala, J.; Bello-Perez, L.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Sansano, M.; Castelló, M.; Heredia, A.; Andrés, A. Protective effect of chitosan on acrylamide formation in model and batter systems. Food Hydrocoll. 2016, 60, 1–6. [Google Scholar] [CrossRef]
- Zyzak, D.; Sanders, R.; Stojanovic, M.; Tallmadge, D.; Eberhart, B.; Ewald, D.; Villagran, M. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Becalski, A.; Lau, B.; Lewis, D.; Seaman, S. Acrylamide in foods: Occurrence, sources, and modeling. J. Agric. Food Chem. 2003, 51, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Rosén, J.; Hellenäs, K. Analysis of acrylamide in cooked foods by liquid chromatography tandem mass spectrometry. Analyst 2002, 127, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Koutsidis, G. Investigations on the effect of antioxidant type and concentration and model system matrix on acrylamide formation in model Maillard reaction systems. Food Chem. 2016, 197, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Sansano, M.; Heredia, A.; Peinado, I.; Andrés, A. Dietary acrylamide: What happens during digestion. Food Chem. 2017, 237, 58–64. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Saeidnia, S.; Abdollahi, M. Role of antioxidants and phytochemicals on acrylamide mitigation from food and reducing its toxicity. J. Food Sci. Technol. 2015, 52, 3169–3186. [Google Scholar] [CrossRef]
- Jin, C.; Wu, X.; Zhang, Y. Relationship between antioxidants and acrylamide formation: A review. Food Res. Int. 2013, 51, 611–620. [Google Scholar] [CrossRef]
- Jiang, D.; Chiaro, C.; Maddali, P.; Prabhu, K.; Peterson, D. Identification of hydroxycinnamic acid− maillard reaction products in low-moisture baking model systems. J. Agric. Food Chem. 2009, 57, 9932–9943. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Update on acrylamide levels in food from monitoring years 2007 to 2010. EFSA J. 2012, 10, 1–38. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.; Ke, J.; Corke, H. Dietary plant materials reduce acrylamide formation in cookie and starch-based model systems. J. Sci. Food Agric. 2011, 91, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, Y.; Zhang, Y.; Lu, B.; Jin, C.; Wu, X.; Zhang, Y. Study on mitigation of acrylamide formation in cookies by 5 antioxidants. J. Food Sci. 2012, 77, C1144–C1149. [Google Scholar] [CrossRef]
- Sasaki, T.; Sotome, I.; Okadome, H. In vitro starch digestibility and in vivo glucose response of gelatinized potato starch in the presence of non-starch polysaccharides. Starch-Stärke 2015, 67, 415–423. [Google Scholar] [CrossRef]
- Desai, A.; Brennan, M.; Guo, X.; Zeng, X.; Brennan, C. Fish protein and lipid interactions on the digestibility and bioavailability of starch and protein from durum wheat pasta. Molecules 2019, 24, 839. [Google Scholar] [CrossRef]
- Xiao, H.; Lin, Q.; Liu, G.; Wu, Y.; Tian, W.; Wu, W.; Fu, X. Effect of green tea polyphenols on the gelatinization and retrogradation of rice starches with different amylose contents. J. Med. Plants Res. 2011, 5, 4298–4303. [Google Scholar]
- Dueik, V.; Bouchon, P. Development of polyphenol-enriched vacuum and atmospheric fried matrices: Evaluation of quality parameters and in vitro bioavailability of polyphenols. Food Res. Int. 2016, 88, 166–172. [Google Scholar] [CrossRef]
- Lima, D.; Almeida, D.; Pasquali, M.; Borges, S.; Fook, M.; Lisboa, H. Physical characterization and modeling of chitosan/peg blends for injectable scaffolds. Carbohyd. Polym. 2018, 189, 238–249. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Muy-Rangel, D.; de la Garza, A.L.; Rubio-Carrasco, W.; Pérez-Meza, B.; Araujo-Chapa, A.P.; Gutiérrez-Álvarez, K.A.; Urías-Orona, V. Dietary Fiber from Chickpea (Cicer arietinum) and Soybean (Glycine max) Husk Byproducts as Baking Additives: Functional and Nutritional Properties. Molecules 2019, 24, 991. [Google Scholar] [CrossRef] [PubMed]
- A.O.A.C. Official Methods of Analysis Washington: Association of Official Analytical Chemist. Available online: https://www.aoac.org/aoac_prod_imis/AOAC/Publications/Official_Methods_of_Analysis/AOAC_Member/Pubs/OMA/AOAC_Official_Methods_of_Analysis.aspx (accessed on 20 September 2017).
- Skendi, A.; Biliaderis, C.; Papageorgiou, M.; Izydorczyk, M. Effects of two barley β-glucan isolates on wheat flour dough and bread properties. Food Chem. 2010, 119, 1159–1167. [Google Scholar] [CrossRef]
- Lazaridou, A.; Vouris, D.; Zoumpoulakis, P.; Biliaderis, C. Physicochemical properties of jet milled wheat flours and doughs. Food Hydrocoll. 2018, 80, 111–121. [Google Scholar] [CrossRef]
- Pacetti, D.; Gil, E.; Frega, N.; Álvarez, L.; Dueñas, P.; Garzón, A.; Lucci, P. Acrylamide levels in selected Colombian foods. Food Addit. Contam. Part B 2015, 8, 99–105. [Google Scholar] [CrossRef]
- Toutounji, M.; Farahnaky, A.; Santhakumar, A.; Oli, P.; Butardo, V.; Blanchard, C. Intrinsic and extrinsic factors affecting rice starch digestibility. Trends Food Sci. Technol. 2019, 88, 10–22. [Google Scholar] [CrossRef]
- Gouseti, O.; Lovegrove, A.; Kosik, O.; Fryer, P.; Mills, C.; Gates, F.; Tucker, G.; Latty, C.; Shewry, P.; Bakalis, S. Exploring the Role of Cereal Dietary Fiber in Digestion. J. Agric. Food Chem. 2019, 2019. 67, 8419–8424. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| Components | Parameters * | |
|---|---|---|
| Water-Holding Capacity (g Water/g Dry Solids) | Apparent Viscosity (mPa·s) | |
| 3000 rpm (704× g) for 15 min | Solutions at 5% and 30 °C | |
| Inulin (IN) | 2.49 ± 0.23 b | 2.76 ± 0.47 a |
| Polydextrose (PD) | 1.96 ± 0.18 b | 5.49 ± 0.51 c |
| Partially hydrolyzed guar gum (PHGG) | 2.36 ± 0.19 b | 8.74 ± 0.68 d |
| Wheat starch | 1.85 ± 0.12 a | 3.46 ± 0.68 b |
| Ingredients | |||||||
|---|---|---|---|---|---|---|---|
| Product Code * | Wheat Gluten (g/100g Dry Solids) | Native Starch (g/100g Dry Solids) | SDF (g/100g Dry Solids of Starch) | GTP (g/100g Dry Solid of Starch) | Moisture (% Wet Basis) | ||
| IN | PD | PHGG | |||||
| GS | 12 | 88 | - | - | - | - | 40 |
| GS-IN | 12 | 80.5 | 7.5 | - | - | - | 38 |
| GS-PD | 12 | 80.5 | - | 7.5 | - | - | 38 |
| GS-PHGG | 12 | 80.5 | - | - | 7.5 | - | 38 |
| GS-GTP | 12 | 86.5 | - | - | - | 1.0 | 42 |
| GS-IN-GTP | 12 | 79.5 | 7.5 | - | - | 1.0 | 40 |
| GS-PD-GTP | 12 | 79.5 | - | 7.5 | - | 1.0 | 40 |
| GS-PHGG-GTP | 12 | 79.5 | - | - | 7.5 | 1.0 | 40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, J.D.; Dueik, V.; Carré, D.; Bouchon, P. Effect of the Addition of Soluble Dietary Fiber and Green Tea Polyphenols on Acrylamide Formation and In Vitro Starch Digestibility in Baked Starchy Matrices. Molecules 2019, 24, 3674. https://doi.org/10.3390/molecules24203674
Torres JD, Dueik V, Carré D, Bouchon P. Effect of the Addition of Soluble Dietary Fiber and Green Tea Polyphenols on Acrylamide Formation and In Vitro Starch Digestibility in Baked Starchy Matrices. Molecules. 2019; 24(20):3674. https://doi.org/10.3390/molecules24203674
Chicago/Turabian StyleTorres, José David, Verónica Dueik, David Carré, and Pedro Bouchon. 2019. "Effect of the Addition of Soluble Dietary Fiber and Green Tea Polyphenols on Acrylamide Formation and In Vitro Starch Digestibility in Baked Starchy Matrices" Molecules 24, no. 20: 3674. https://doi.org/10.3390/molecules24203674
APA StyleTorres, J. D., Dueik, V., Carré, D., & Bouchon, P. (2019). Effect of the Addition of Soluble Dietary Fiber and Green Tea Polyphenols on Acrylamide Formation and In Vitro Starch Digestibility in Baked Starchy Matrices. Molecules, 24(20), 3674. https://doi.org/10.3390/molecules24203674