Exploring the Phytochemical Landscape of the Early-Diverging Flowering Plant Amborella trichopoda Baill.
Abstract
:1. Introduction
2. Results
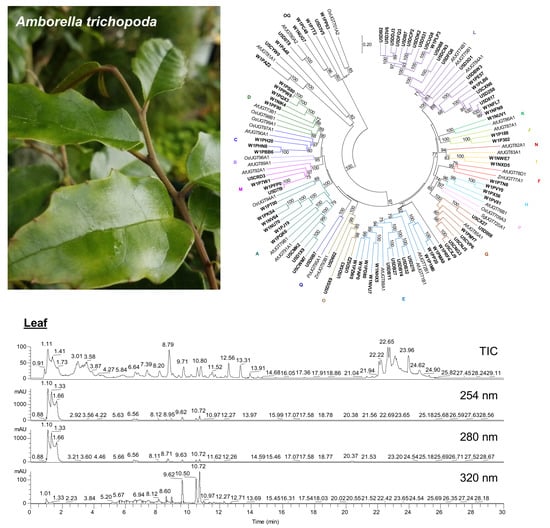
2.1. Abundant Phenolic Compounds with Glycosylation and Methylation Modifications in Amborella Leaves
2.2. Diverse Metabolites in Amborella Stems and Roots
2.3. Large Families of UDP-Dependent Glycosyltransferases (UGTs) and O-Methyltransferases (OMTs) in the Amborella Genome
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Metabolite Analysis
4.3. Metabolite Identification
4.4. Phylogenetic Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drew, B.T.; Ruhfel, B.R.; Smith, S.A.; Moore, M.J.; Briggs, B.G.; Gitzendanner, M.A.; Soltis, P.S.; Soltis, D.E. Another look at the root of the angiosperms reveals a familiar tale. Syst. Biol. 2014, 63, 368–382. [Google Scholar] [CrossRef]
- Young, D.A. Leaf flavonoids of Amborella trichopoda. Biochem. Syst. Ecol. 1982, 10, 21–22. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J. Improving the flavor of fresh fruits: Genomics, biochemistry, and biotechnology. New Phytol. 2010, 187, 44–56. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Salim, V.; Atsumi, S.M.; Yu, F. Mining the biodiversity of plants: A revolution in the making. Science 2012, 336, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Koyama, T. Enzymatic aspects of isoprenoid chain elongation. Chem. Rev. 1998, 98, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef]
- Dixon, R.A.; Strack, D. Phytochemistry meets genome analysis, and beyond. Phytochemistry 2003, 62, 815–816. [Google Scholar] [CrossRef]
- Tian, L.; Pang, Y.; Dixon, R. Biosynthesis and genetic engineering of proanthocyanidins and (iso) flavonoids. Phytochem. Rev. 2008, 7, 445–465. [Google Scholar] [CrossRef]
- Hughes, J.; Hughes, M. Multiple secondary plant product UDP-glucose glucosyltransferase genes expressed in cassava (Manihot esculenta Crantz) cotyledons. DNA Seq. 1994, 5, 41–49. [Google Scholar] [CrossRef]
- D’Auria, J.C.; Chen, F.; Pichersky, E. The SABATH family of MTS in Arabidopsis thaliana and other plant species. In Recent Advances in Phytochemistry; Romeo, J.T., Ed.; Elsevier: Amesterdam, The Netherlands, 2003; Volume 37, pp. 253–283. [Google Scholar]
- Amborella Genome Project. The Amborella genome and the evolution of flowering plants. Science 2013, 342, 1241089. [Google Scholar] [CrossRef] [PubMed]
- Paquette, S.; Møller, B.L.; Bak, S. On the origin of family 1 plant glycosyltransferases. Phytochemistry 2003, 62, 399–413. [Google Scholar] [CrossRef]
- Kapteyn, J.; Qualley, A.V.; Xie, Z.; Fridman, E.; Dudareva, N.; Gang, D.R. Evolution of cinnamate/p-coumarate carboxyl methyltransferases and their role in the biosynthesis of methylcinnamate. Plant cell 2007, 19, 3212–3229. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Marchand, P.A.; Griswold, J.; Lewis, N.G.; Ryan, C.A. Accumulation of feruloyltyramine and p-coumaroyltyramine in tomato leaves in response to wounding. Phytochemistry 1998, 47, 659–664. [Google Scholar] [CrossRef]
- Newman, M.-A.; von Roepenack-Lahaye, E.; Parr, A.; Daniels, M.J.; Dow, J.M. Induction of hydroxycinnamoyl-tyramine conjugates in pepper by Xanthomonas campestris, a plant defense response activated by hrp gene-dependent and hrp gene-independent mechanisms. Mol. Plant Microbe Interact. 2001, 14, 785–792. [Google Scholar] [CrossRef]
- Liu, C.-J.; Deavours, B.E.; Richard, S.B.; Ferrer, J.-L.; Blount, J.W.; Huhman, D.; Dixon, R.A.; Noel, J.P. Structural basis for dual functionality of isoflavonoid O-methyltransferases in the evolution of plant defense responses. Plant cell 2006, 18, 3656–3669. [Google Scholar] [CrossRef]
- Jackson, R.G.; Lim, E.-K.; Li, Y.; Kowalczyk, M.; Sandberg, G.; Hoggett, J.; Ashford, D.A.; Bowles, D.J. Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J. Biol. Chem. 2001, 276, 4350–4356. [Google Scholar] [CrossRef]
- Lim, E.; Li, Y.; Parr, A.; Jackson, R.; Ashford, D.; Bowles, D. Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. J. Biol. Chem. 2001, 276, 4344–4349. [Google Scholar] [CrossRef]
- Lim, E.; Doucet, C.; Li, Y.; Elias, L.; Worrall, D.; Spencer, S.; Ross, J.; Bowles, D. The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J. Biol. Chem. 2002, 277, 586–592. [Google Scholar] [CrossRef]
- Lim, E.-K.; Baldauf, S.; Li, Y.; Elias, L.; Worrall, D.; Spencer, S.P.; Jackson, R.G.; Taguchi, G.; Ross, J.; Bowles, D.J. Evolution of substrate recognition across a multigene family of glycosyltransferases in Arabidopsis. Glycobiology 2003, 13, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Brazier-Hicks, M.; Gershater, M.; Dixon, D.; Edwards, R. Substrate specificity and safener inducibility of the plant UDP-glucose-dependent family 1 glycosyltransferase super-family. Plant Biotechnol. J. 2018, 16, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Itkin, M.; Davidovich-Rikanati, R.; Cohen, S.; Portnoy, V.; Doron-Faigenboim, A.; Oren, E.; Freilich, S.; Tzuri, G.; Baranes, N.; Shen, S.; et al. The biosynthetic pathway of the nonsugar, high-intensity sweetener mogroside V from Siraitia grosvenorii. Proc. Natl. Acad. Sci. USA 2016, 113, E7619–E7628. [Google Scholar] [CrossRef] [PubMed]
- Grubb, C.D.; Zipp, B.J.; Kopycki, J.; Schubert, M.; Quint, M.; Lim, E.-K.; Bowles, D.J.; Pedras, M.S.C.; Abel, S. Comparative analysis of Arabidopsis UGT74 glucosyltransferases reveals a special role of UGT74C1 in glucosinolate biosynthesis. Plant J. 2014, 79, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, K.; Dong, L.; Navrot, N.; Schneider, T.; Burlat, V.; Pollier, J.; Woittiez, L.; van der Krol, S.; Lugan, R.; Ilc, T.; et al. The seco-iridoid pathway from Catharanthus roseus. Nat. Commun. 2014, 5, 3606. [Google Scholar] [CrossRef]
- Ibdah, M.; Zhang, X.-H.; Schmidt, J.; Vogt, T. A novel Mg2+-dependent O-Methyltransferase in the phenylpropanoid metabolism of Mesembryanthemum crystallinum. J. Biol. Chem. 2003, 278, 43961–43972. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Gold, N.D.; Martin, V.J.J.; Wollenweber, E.; Ibrahim, R.K. Sequential O-methylation of tricetin by a single gene product in wheat. Biochim. Biophys. Acta 2006, 1760, 1115–1124. [Google Scholar] [CrossRef]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef]
- Bowles, D.; Isayenkova, J.; Lim, E.; Poppenberger, B. Glycosyltransferases: Managers of small molecules. Curr. Opin. Plant Biol. 2005, 8, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.E.; Wu, S.; Tian, L. PgUGT95B2 preferentially metabolizes flavones/flavonols and has evolved independently from flavone/flavonol UGTs identified in Arabidopsis thaliana. Phytochemistry 2019, 157, 184–193. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass. Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, D. CASMI 2016: A manual approach for dereplication of natural products using tandem mass spectrometry. Phytochem. Lett. 2017, 21, 292–296. [Google Scholar] [CrossRef]
- Naz, S.; Gallart-Ayala, H.; Reinke, S.N.; Mathon, C.; Blankley, R.; Chaleckis, R.; Wheelock, C.E. Development of a liquid chromatography–high resolution mass spectrometry metabolomics method with high specificity for metabolite identification using all ion fragmentation acquisition. Anal. Chem. 2017, 89, 7933–7942. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.-Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucl. Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef]
- Edgar, R. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
Sample Availability: Samples of some of the phenolic compounds are available from the authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Wilson, A.E.; Chang, L.; Tian, L. Exploring the Phytochemical Landscape of the Early-Diverging Flowering Plant Amborella trichopoda Baill. Molecules 2019, 24, 3814. https://doi.org/10.3390/molecules24213814
Wu S, Wilson AE, Chang L, Tian L. Exploring the Phytochemical Landscape of the Early-Diverging Flowering Plant Amborella trichopoda Baill. Molecules. 2019; 24(21):3814. https://doi.org/10.3390/molecules24213814
Chicago/Turabian StyleWu, Sheng, Alexander E. Wilson, Lijing Chang, and Li Tian. 2019. "Exploring the Phytochemical Landscape of the Early-Diverging Flowering Plant Amborella trichopoda Baill." Molecules 24, no. 21: 3814. https://doi.org/10.3390/molecules24213814
APA StyleWu, S., Wilson, A. E., Chang, L., & Tian, L. (2019). Exploring the Phytochemical Landscape of the Early-Diverging Flowering Plant Amborella trichopoda Baill. Molecules, 24(21), 3814. https://doi.org/10.3390/molecules24213814