Insights into Lignan Composition and Biosynthesis in Stinging Nettle (Urtica dioica L.)
Abstract
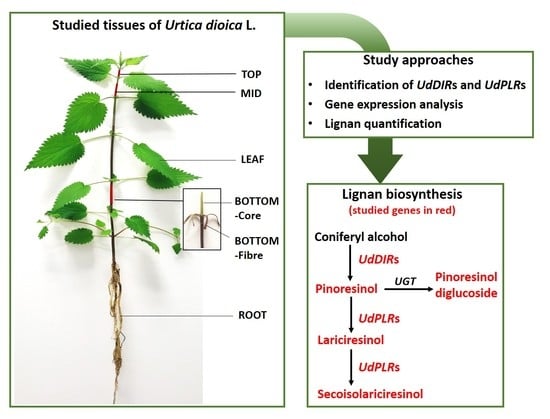
:1. Introduction
2. Results
2.1. Identification of UdDIRs
2.2. Identification of UdPLRs
2.3. Targeted Quantification of Lignans in Different Tissues of Stinging Nettle
2.4. Gene Expression Analysis of DIRs and PLRs in Different Tissues
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Gene Identification and Phylogenetic Analysis
4.3. Lignan Extraction and Quantification
4.4. Total RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR (RT-qPCR)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akiyama, K.; Yamauchi, S.; Nakato, T.; Maruyama, M.; Sugahara, T.; Kishida, T. Antifungal activity of tetra-substituted tetrahydrofuran lignan,(−)-virgatusin and its structure-activity relationship. Biosci. Biotechnol. Biochem. 2007, 71, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Gang, D.R.; Kasahara, H.; Xia, Z.-Q.; Vander Mijnsbrugge, K.; Bauw, G.; Boerjan, W.; Van Montagu, M.; Davin, L.B.; Lewis, N.G. Evolution of plant defense mechanisms relationships of phenylcoumaran benzylic ether reductases to pinoresinol-lariciresinol and isoflavone reductases. J. Biol. Chem. 1999, 274, 7516–7527. [Google Scholar] [CrossRef] [PubMed]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide analysis of phenylpropanoid defence pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Liang, Z.; Zhao, C. New lignans and their biological activities. Chem. Biodiver. 2014, 11, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, T.; Irene, T. Polyphenols encapsulation–application of innovation technologies to improve stability of natural products. Phys. Sci. Rev. 2016, 1. [Google Scholar] [CrossRef]
- Francišković, M.; Gonzalez-Pérez, R.; Orčić, D.; Sánchez de Medina, F.; Martínez-Augustin, O.; Svirčev, E.; Simin, N.; Mimica-Dukić, N. Chemical Composition and Immuno-Modulatory Effects of Urtica dioica L.(Stinging Nettle) Extracts. Phytother. Res. 2017, 31, 1183–1191. [Google Scholar] [CrossRef]
- Schöttner, M.; Ganßer, D.; Spiteller, G. Lignans from the roots of Urtica dioica and their metabolites bind to human sex hormone binding globulin (SHBG). Planta Med. 1997, 63, 529–532. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Mohajerani, R. Phytochemistry and pharmacologic properties of Urtica dioica L. J. Med. Plant Res. 2012, 6, 5714–5719. [Google Scholar]
- Chrubasik, S.; Enderlein, W.; Bauer, R.; Grabner, W. Evidence for antirheumatic effectiveness of Herba Urticae dioicae in acute arthritis: A pilot study. Phytomedicine 1997, 4, 105–108. [Google Scholar] [CrossRef]
- Legssyer, A.; Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Tahri, A.; Serhrouchni, M.; Hoerter, J.; Fischmeister, R. Cardiovascular effects of Urtica dioica L. in isolated rat heart and aorta. Phytother. Res. 2002, 16, 503–507. [Google Scholar] [CrossRef]
- Roschek, B., Jr.; Fink, R.C.; McMichael, M.; Alberte, R.S. Nettle extract (Urtica dioica) affects key receptors and enzymes associated with allergic rhinitis. Phytother. Res. 2009, 23, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Vajic, U.-J.; Grujic-Milanovic, J.; Miloradovic, Z.; Jovovic, D.; Ivanov, M.; Karanovic, D.; Savikin, K.; Bugarski, B.; Mihailovic-Stanojevic, N. Urtica dioica L. leaf extract modulates blood pressure and oxidative stress in spontaneously hypertensive rats. Phytomedicine 2018, 46, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Tahri, A.; Yamani, S.; Legssyer, A.; Aziz, M.; Mekhfi, H.; Bnouham, M.; Ziyyat, A. Acute diuretic, natriuretic and hypotensive effects of a continuous perfusion of aqueous extract of Urtica dioica in the rat. J. Ethnopharmacol. 2000, 73, 95–100. [Google Scholar] [CrossRef]
- Schöttner, M.; Reiner, J.; Tayman, F.S. (+)-neo-olivil from roots of Urtica dioica. Phytochemistry 1997, 46, 1107–1109. [Google Scholar] [CrossRef]
- Kraus, R.; Spiteller, G. Phenolic compounds from roots of Urtica dioica. Phytochemistry 1990, 29, 1653–1659. [Google Scholar] [CrossRef]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.S. Lignans neolignans and related compounds. Nat. Prod. Rep. 1993, 10, 1–28. [Google Scholar] [CrossRef]
- Markulin, L.; Corbin, C.; Renouard, S.; Drouet, S.; Gutierrez, L.; Mateljak, I.; Auguin, D.; Hano, C.; Fuss, E.; Lainé, E. Pinoresinol–lariciresinol reductases, key to the lignan synthesis in plants. Planta 2019, 249, 1695–1714. [Google Scholar] [CrossRef]
- Umezawa, T. Diversity in lignan biosynthesis. Phytochem. Rev. 2003, 2, 371–390. [Google Scholar] [CrossRef]
- Davin, L.B.; Wang, H.-B.; Crowell, A.L.; Bedgar, D.L.; Martin, D.M.; Sarkanen, S.; Lewis, N.G. Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science 1997, 275, 362–367. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Kim, K.W.; Lee, C.; Yang, H.; Rúbel, O.; Bowen, B.P.; Davin, L.B.; Lewis, N.G. Dirigent protein-mediated lignan and cyanogenic glucoside formation in flax seed: Integrated omics and MALDI mass spectrometry imaging. J. Nat. Prod. 2015, 78, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.G.; Jancsik, S.; Bohlmann, J. Dirigent proteins in conifer defense II: Extended gene discovery, phylogeny and constitutive and stress-induced gene expression in spruce (Picea spp.). Phytochemistry 2007, 68, 1975–1991. [Google Scholar] [CrossRef] [PubMed]
- Vassão, D.G.; Kim, K.-W.; Davin, L.B.; Lewis, N.G. Lignans (neolignans) and allyl/propenyl phenols: Biogenesis, structural biology and biological/human health considerations. Chem. Biol. 2010, 1, 815–928. [Google Scholar]
- Hosmani, P.S.; Kamiya, T.; Danku, J.; Naseer, S.; Geldner, N.; Guerinot, M.L.; Salt, D.E. Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based Casparian strip in the root. Proc. Natl. Acad. Sci. USA 2013, 110, 14498–14503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davin, L.B.; Jourdes, M.; Patten, A.M.; Kim, K.-W.; Vassão, D.G.; Lewis, N.G. Dissection of lignin macromolecular configuration and assembly: Comparison to related biochemical processes in allyl/propenyl phenol and lignan biosynthesis. Nat. Prod. Rep. 2008, 25, 1015–1090. [Google Scholar] [CrossRef]
- Jin-long, G.; Li-ping, X.; Jing-ping, F.; Ya-chun, S.; Hua-ying, F.; You-xiong, Q.; Jing-sheng, X. A novel dirigent protein gene with highly stem-specific expression from sugarcane, response to drought, salt and oxidative stresses. Plant Cell Rep. 2012, 31, 1801–1812. [Google Scholar] [CrossRef]
- Behr, M.; Legay, S.; Hausman, J.-F.; Guerriero, G. Analysis of cell wall-related genes in organs of Medicago sativa L. under different abiotic stresses. Inter. J. Mol. Sci. 2015, 16, 16104–16124. [Google Scholar] [CrossRef]
- Fujita, M.; Gang, D.R.; Davin, L.B.; Lewis, N.G. Recombinant pinoresinol-lariciresinol reductases from western red cedar (Thuja plicata) catalyze opposite enantiospecific conversions. J. Biol. Chem. 1999, 274, 618–627. [Google Scholar] [CrossRef]
- Hemmati, S.; von Heimendahl, C.B.; Klaes, M.; Alfermann, A.W.; Schmidt, T.J.; Fuss, E. Pinoresinol-lariciresinol reductases with opposite enantiospecificity determine the enantiomeric composition of lignans in the different organs of Linum usitatissimum L. Planta Med. 2010, 76, 928–934. [Google Scholar] [CrossRef]
- Xu, X.; Backes, A.; Legay, S.; Berni, R.; Faleri, C.; Gatti, E.; Hausman, J.F.; Cai, G.; Guerriero, G. Cell wall composition and transcriptomics in stem tissues of stinging nettle (Urtica dioica L.): Spotlight on a neglected fibre crop. Plant Direct 2019, 3, e00151. [Google Scholar] [CrossRef]
- Min, T.; Kasahara, H.; Bedgar, D.L.; Youn, B.; Lawrence, P.K.; Gang, D.R.; Halls, S.C.; Park, H.; Hilsenbeck, J.L.; Davin, L.B. Crystal structures of pinoresinol-lariciresinol and phenylcoumaran benzylic ether reductases and their relationship to isoflavone reductases. J. Biol. Chem. 2003, 278, 50714–50723. [Google Scholar] [CrossRef] [PubMed]
- von Heimendahl, C.B.; Schäfer, K.M.; Eklund, P.; Sjöholm, R.; Schmidt, T.J.; Fuss, E. Pinoresinol–lariciresinol reductases with different stereospecificity from Linum album and Linum usitatissimum. Phytochemistry 2005, 66, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Bayindir, U.; Alfermann, A.W.; Fuss, E. Hinokinin biosynthesis in Linum corymbulosum Reichenb. Plant J. 2008, 55, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Gang, D.R.; Davin, L.B.; Bedgar, D.L.; Chu, A.; Lewis, N.G. (+)-Pinoresinol/(+)-lariciresinol reductase from Forsythia intermedia. Protein purification, cDNA cloning, heterologous expression and comparison to isoflavone reductase. J. Biol. Chem. 1996, 271, 29473–29482. [Google Scholar] [CrossRef]
- Wankhede, D.P.; Biswas, D.K.; Rajkumar, S.; Sinha, A.K. Expressed sequence tags and molecular cloning and characterization of gene encoding pinoresinol/lariciresinol reductase from Podophyllum hexandrum. Protoplasma 2013, 250, 1239–1249. [Google Scholar] [CrossRef]
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Xing, D.; Ma, G.; Dai, X.; Gao, L.; Xia, T. A variable loop involved in the substrate selectivity of pinoresinol/lariciresinol reductase from Camellia sinensis. Phytochemistry 2019, 162, 1–9. [Google Scholar] [CrossRef]
- Song, M.; Peng, X. Genome-Wide Identification and Characterization of DIR Genes in Medicago truncatula. Biochem. Genet. 2019, 1–20. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, S.; Jiang, Y.; Hu, C.; Zhang, X.; Cao, X.; Xu, Z.; Gao, X.; Li, L.; Zhu, J. Genome-wide analysis and environmental response profiling of dirigent family genes in rice (Oryza sativa). Genes Genom. 2017, 39, 47–62. [Google Scholar] [CrossRef]
- Corbin, C.; Drouet, S.; Markulin, L.; Auguin, D.; Lainé, É.; Davin, L.B.; Cort, J.R.; Lewis, N.G.; Hano, C. A genome-wide analysis of the flax (Linum usitatissimum L.) dirigent protein family: From gene identification and evolution to differential regulation. Plant Mol. Biol. 2018, 97, 73–101. [Google Scholar] [CrossRef]
- Kim, M.K.; Jeon, J.-H.; Fujita, M.; Davin, L.B.; Lewis, N.G. The western red cedar (Thuja plicata) 8-8′ DIRIGENT family displays diverse expression patterns and conserved monolignol coupling specificity. Plant Mol. Biol. 2002, 49, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.-Q.; Costa, M.A.; Proctor, J.; Davin, L.B.; Lewis, N.G. Dirigent-mediated podophyllotoxin biosynthesis in Linum flavum and Podophyllum peltatum. Phytochemistry 2000, 55, 537–549. [Google Scholar] [CrossRef]
- Li, N.; Zhao, M.; Liu, T.; Dong, L.; Cheng, Q.; Wu, J.; Wang, L.; Chen, X.; Zhang, C.; Lu, W. A novel soybean dirigent gene GmDIR22 contributes to promotion of lignan biosynthesis and enhances resistance to Phytophthora sojae. Front. Plant Sci. 2017, 8, 1185. [Google Scholar] [CrossRef] [PubMed]
- Ayres, D.C.; Loike, J.D. Lignans: Chemical, Biological and Clinical Properties; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Palter, R.; Lundin, R.; Haddon, W. A cathartic lignan glycoside isolated from Cart. Tinctorus. Phytochem. 1972, 11, 2871–2874. [Google Scholar] [CrossRef]
- Katsuzaki, H.; Kawasumi, M.; Kawakishi, S.; Osawa, T. Structure of novel antioxidative lignan glucosides isolated from sesame seed. Biosci. Biotechnol. Biochem. 1992, 56, 2087–2088. [Google Scholar] [CrossRef]
- Katsuzaki, H.; Kawakishi, S.; Osawa, T. Sesaminol glucosides in sesame seeds. Phytochemistry 1994, 35, 773–776. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Andersson, R.E.; Kamal-Eldin, A. HPLC analysis of sesaminol glucosides in sesame seeds. J. Agric. Food Chem. 2006, 54, 633–638. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Andersson, R.E.; Kamal-Eldin, A. Characterization and analysis of sesamolinol diglucoside in sesame seeds. Biosci. Biotechnol. Biochem. 2006, 70, 1478–1481. [Google Scholar] [CrossRef]
- Noguchi, A.; Fukui, Y.; Iuchi-Okada, A.; Kakutani, S.; Satake, H.; Iwashita, T.; Nakao, M.; Umezawa, T.; Ono, E. Sequential glucosylation of a furofuran lignan,(+)-sesaminol, by Sesamum indicum UGT71A9 and UGT94D1 glucosyltransferases. Plant J. 2008, 54, 415–427. [Google Scholar] [CrossRef]
- Yamauchi, S.; Ichikawa, H.; Nishiwaki, H.; Shuto, Y. Evaluation of plant growth regulatory activity of furofuran lignan bearing a 7, 9′: 7′, 9-Diepoxy structure using optically pure (+)-and (−)-Enantiomers. J. Agric. Food Chem. 2015, 63, 5224–5228. [Google Scholar] [CrossRef]
- Cutillo, F.; D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Zarrelli, A. Lignans and neolignans from Brassica fruticulosa: Effects on seed germination and plant growth. J. Agric. Food Chem. 2003, 51, 6165–6172. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; Kumamoto, M.; Ochi, Y.; Nishiwaki, H.; Shuto, Y. Structure–Plant Growth Inhibitory Activity Relationship of Lariciresinol. J. Agric. Food Chem. 2013, 61, 12297–12306. [Google Scholar] [CrossRef] [PubMed]
- Sih, C.J.; Ravikumar, P.; Huang, F.-C.; Buckner, C.; Whitlock, H., Jr. Isolation and synthesis of pinoresinol diglucoside, a major antihypertensive principle of Tu-Chung (Eucommia ulmoides, Oliver). J. Am. Chem. Soc. 1976, 98, 5412–5413. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-F.; Wu, W.-H.; Zhou, Y.-J.; Yan, J.; Yang, G.-P.; Ouyang, D.-S. Antihypertensive effect of Eucommia ulmoides Oliv. extracts in spontaneously hypertensive rats. J. Ethnopharmacol. 2010, 129, 238–243. [Google Scholar] [CrossRef]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Liu, L.; Gao, Z.; Che, J.; Shao, D.; Liu, Y. Bioconversion of pinoresinol diglucoside and pinoresinol from substrates in the phenylpropanoid pathway by resting cells of Phomopsis sp. XP-8. PLoS ONE 2015, 10, e0137066. [Google Scholar]
- Zhang, Y.; Shi, J.; Gao, Z.; Yangwu, R.; Jiang, H.; Che, J.; Liu, Y. Production of pinoresinol diglucoside, pinoresinol monoglucoside and pinoresinol by Phomopsis sp. XP-8 using mung bean and its major components. Appl. Microbiol. Biotechnol. 2015, 99, 4629–4643. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Gao, Z.; Che, J.; Shao, D.; Liu, Y. Comparison of pinoresinol diglucoside production by Phomopsis sp. XP-8 in different media and the characterisation and product profiles of the cultivation in mung bean. J. Sci. Food Agric. 2016, 96, 4015–4025. [Google Scholar] [CrossRef]
- Nakatsubo, T.; Mizutani, M.; Suzuki, S.; Hattori, T.; Umezawa, T. Characterization of Arabidopsis thaliana pinoresinol reductase, a new type of enzyme involved in lignan biosynthesis. J. Biol. Chem. 2008, 283, 15550–15557. [Google Scholar] [CrossRef]
- Huis, R.; Morreel, K.; Fliniaux, O.; Lucau-Danila, A.; Fénart, S.; Grec, S.; Neutelings, G.; Chabbert, B.; Mesnard, F.; Boerjan, W. Natural hypolignification is associated with extensive oligolignol accumulation in flax stems. Plant Physiol. 2012, 158, 1893–1915. [Google Scholar] [CrossRef]
- Chou, K.C. Progresses in predicting post-translational modification. Int. J. Pept. Res. Ther. 2019, 1–16. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, S.; Long, R.; Chou, K.C. iRSpot-EL: Identify recombination spots with an ensemble learning approach. Bioinformatics 2017, 33, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.C. Impacts of bioinformatics to medicinal chemistry. Med. Chem. 2015, 11, 218–234. [Google Scholar] [CrossRef]
- Bacci, L.; Baronti, S.; Predieri, S.; Di Virgilio, N. Fiber yield and quality of fiber nettle (Urtica dioica L.) cultivated in Italy. Ind. Crop. Prod. 2009, 29, 480–484. [Google Scholar] [CrossRef]
- Backes, A.; Behr, M.; Xu, X.; Gatti, E.; Legay, S.; Predieri, S.; Hausman, J.-F.; Deyholos, M.K.; Cai, G.; Guerriero, G. Sucrose synthase gene expression analysis in the fibre nettle (Urtica dioica L.) cultivar “clone 13”. Ind. Crop. Prod. 2018, 123, 315–322. [Google Scholar] [CrossRef]
- McWilliam, H.; Li, W.; Uludag, M.; Squizzato, S.; Park, Y.M.; Buso, N.; Cowley, A.P.; Lopez, R. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013, 41, W597–W600. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Behr, M.; Sergeant, K.; Leclercq, C.C.; Planchon, S.; Guignard, C.; Lenouvel, A.; Renaut, J.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Insights into the molecular regulation of monolignol-derived product biosynthesis in the growing hemp hypocotyl. BMC Plant Biol. 2018, 18, 1. [Google Scholar] [CrossRef]
- Milder, I.E.J.; Arts, I.C.W.; Venema, D.P.; Lasaroms, J.J.P.; Wähälä, K.; Hollman, P.C.H. Optimization of a Liquid Chromatography−Tandem Mass Spectrometry Method for Quantification of the Plant Lignans Secoisolariciresinol, Matairesinol, Lariciresinol and Pinoresinol in Foods. J. Agric. Food Chem. 2004, 52, 4643–4651. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Hoon, M.J.; Imoto, S.; Nolan, J.; Miyano, S. Open source clustering software. Bioinformatics 2004, 20, 1453–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |







| Nomenclature | Transcript ID | ORF Length | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Efficiency (%) | R2 |
|---|---|---|---|---|---|---|
| UdDIR1 * | contig_12966 | 191 | TCTCATGGTCCTCAACTACGTC | TTCCGTCCCAATATGCTGAG | 99.474 | 0.99 |
| UdDIR2 * | contig_14063 | 192 | TCTCAAGCTCACACGCAAAC | AGAGCTTTTCTCTGCGGAGTC | 91.93 | 0.997 |
| UdDIR3 | contig_22204 | 186 | ||||
| UdDIR4 # | contig_23037 | 103 | ||||
| UdDIR5 * | contig_24527 | 201 | GTCATCAAGCCATGCAAGAG | TCTTGAGGTTGTGACCGTTG | 103.679 | 0.99 |
| UdDIR6 | contig_24857 | 178 | ||||
| UdDIR7 *,# | contig_28042 | 143 | ACGTAGTTCTGGACCATGAGG | ATTATCGACGACCCGTTGAC | 90.92 | 0.997 |
| UdDIR8 # | contig_28614 | 135 | ||||
| UdDIR9 * | contig_28699 | 191 | GGCCAAATCAAAGGAGACAG | ACCCCGTTTTCGATAAGGTC | 94.912 | 0.988 |
| UdDIR10 # | contig_32790 | 85 | ||||
| UdDIR11 *# | contig_34554 | 160 | GGGAAACCTTCATGATCGAC | TGACCATGAGTAGGGCAATG | 96.397 | 0.998 |
| UdDIR12 * | contig_34733 | 189 | GGGCACTTTGAACGTAATGG | TCCTTGGTAAGTGTCGGTCTG | 94.462 | 0.998 |
| UdDIR13 *,# | contig_34949 | 183 | TCACAGCGTCGAAAGACAAC | TCACGCGTCATCTTGTCATC | 94.294 | 0.995 |
| UdDIR14 | contig_7375 | 203 | ||||
| UdPLR1 *,# | contig_26577 | 308 | CGCCTCTTTCGAAGACAAAG | AGAAGGATGTGATGGGTTCG | 94.212 | 0.995 |
| UdPLR2 * | contig_628 | 312 | CTCGTCGAAGGTTCGTTTTC | CCGGCTTCTTTAATGGCTTC | 99.362 | 0.998 |
| UdPLR3 * | contig_10583 | 309 | CGAAAATGGAGGAGCAGAAG | AAGGTGGGATGAGATGATCG | 101.629 | 0.995 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Guignard, C.; Renaut, J.; Hausman, J.-F.; Gatti, E.; Predieri, S.; Guerriero, G. Insights into Lignan Composition and Biosynthesis in Stinging Nettle (Urtica dioica L.). Molecules 2019, 24, 3863. https://doi.org/10.3390/molecules24213863
Xu X, Guignard C, Renaut J, Hausman J-F, Gatti E, Predieri S, Guerriero G. Insights into Lignan Composition and Biosynthesis in Stinging Nettle (Urtica dioica L.). Molecules. 2019; 24(21):3863. https://doi.org/10.3390/molecules24213863
Chicago/Turabian StyleXu, Xuan, Cédric Guignard, Jenny Renaut, Jean-Francois Hausman, Edoardo Gatti, Stefano Predieri, and Gea Guerriero. 2019. "Insights into Lignan Composition and Biosynthesis in Stinging Nettle (Urtica dioica L.)" Molecules 24, no. 21: 3863. https://doi.org/10.3390/molecules24213863
APA StyleXu, X., Guignard, C., Renaut, J., Hausman, J. -F., Gatti, E., Predieri, S., & Guerriero, G. (2019). Insights into Lignan Composition and Biosynthesis in Stinging Nettle (Urtica dioica L.). Molecules, 24(21), 3863. https://doi.org/10.3390/molecules24213863