Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli
Abstract
:1. Introduction
2. Results
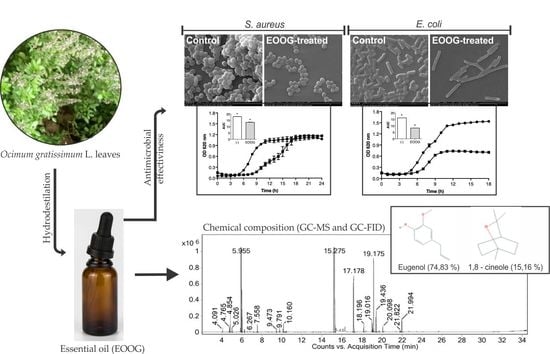
2.1. Extraction and Chemical Composition of the EOOG
2.2. Antibacterial Activity
2.3. Bacterial Growth Curve
2.4. Combination between EOOG and Antibiotics
2.5. Activity on Preformed Biofilm
2.6. Scanning Electron Microscopy
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Extraction
4.3. Chemical Composition of EOOG
4.4. Preparation of Antimicrobial Solutions
4.5. Bacterial Strains and Culture Conditions
4.6. Antimicrobial Activity of EOOG
4.7. Kinetic Growth Assay
4.8. Checkerboard Assay
4.9. Antibiofilm Activity
4.10. Scanning Electron Microscopy
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-resistant bacteria and alternative methods to control them: An overview. Microb. Drug. Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Karam, G.; Chastre, J.; Wilcox, M.H.; Vincent, J.L. Antibiotic strategies in the era of multidrug resistance. Crit. Care 2016, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.S.L.; Tan, L.T.H.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Chuah, L.H.; Ming, L.C.; Khan, T.M.; Lee, L.H.; Goh, B.H. Resveratrol-potential antibacterial agent against foodborne pathogens. Front. Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.K.; Fearn, S.; Allsopp, L.P.; Harrison, F.; Ware, E.; Diggle, S.P.; Filloux, A.; McPhail, D.S.; Bundy, J.G. Visualizing antimicrobials in bacterial biofilms: Three-dimensional biochemical imaging using TOF-SIMS. mSphere 2017, 2, e00211–e00217. [Google Scholar] [CrossRef]
- Makovcova, J.; Babak, V.; Kulich, P.; Masek, J.; Slany, M.; Cincarova, L. Dynamics of mono- and dual-species biofilm formation and interactions between Staphylococcus aureus and Gram-negative bactéria. Microb. Biotechnol. 2017, 10, 819–832. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the Gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rew. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- van Duin, D.; Paterson, D. Multidrug resistant bacteria in the community: Trends and lessons learned. Infect. Dis. Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An overview of the biological effects of some Mediterranean essential oils on human health. Biomed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2016, 22, 70. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2018, 59, 2467–2480. [Google Scholar] [CrossRef] [PubMed]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer properties of essential oils and other natural products. Evid. Based Complement. Alternat. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Pistelli, L.; Mancianti, F. Antimicrobial activity of five essential oils against bacteria and fungi responsible for urinary tract infections. Molecules 2018, 23, 1668. [Google Scholar] [CrossRef]
- Tariqa, S.; Wania, S.; Rasoola, W.; Shafia, K.; Bhata, M.A.; Prabhakarb, A.; Shallaa, A.H.; Rathera, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drugresistant microbial pathogens. Microb. Pathog. 2019, 103580, 134. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S.; Sun, P.L.; Luo, S.L. Antibacterial activity and mechanisms of essential oil from Citrus medica L. Var. Sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef]
- Zomorodian, K.; Moein, M.; Pakshir, K.; Karami, F.; Sabahi, Z. Chemical composition and antimicrobial activities of the essential oil from Salvia mirzayanii leaves. Evid. Based Complement. Alternat. Med. 2017, 22, 770–776. [Google Scholar] [CrossRef]
- Porfírio, E.M.; Melo, H.M.; Pereira, A.M.G.; Cavalcante, T.T.A.; Gomes, G.A.; de Carvalho, M.G.; Costa, R.A.; Catunda Júnior, F.E.A. In vitro antibacterial and antibiofilm activity of Lippia alba essential oil, citral, and carvone against Staphylococcus aureus. Sci. World. J. 2017, 2017, 4962707. [Google Scholar] [CrossRef]
- Vasconcelos, S.E.C.B.; Melo, H.M.; Cavalcante, T.T.A.; Catunda Júnior, F.E.A.; de Carvalho, M.G.; Menezes, F.G.R.; de Sousa, O.V.; Costa, R.A. Plectranthus amboinicus essential oil and carvacrol bioactive against planktonic and biofilm of oxacillin- and vancomycinresistant Staphylococcus aureus. BMC Complement. Altern. Med. 2017, 17, 462. [Google Scholar] [CrossRef] [PubMed]
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; de Carvalho, M.G.; Catunda Júnior, F.E.A. Antibacterial and antibiofilm activities of Cinnamomum Sp. essential oil and cinnamaldehyde: Antimicrobial activities. Sci. World J. 2018, 2018, 7405736. [Google Scholar] [CrossRef] [PubMed]
- Lagha, R.; Abdallah, F.B.; AL-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and biofilm inhibitory activity of medicinal plant essential oils against Escherichia coli isolated from UTI patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef] [PubMed]
- Mohr, F.B.M.; Lermen, C.; Gazim, Z.C.; Gonçalves, J.E.; Alberton, O. Antifungal activity, yield, and composition of Ocimum gratissimum essential oil. Genet. Mol. Res. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Plantas Medicinais de Interesse ao SUS–Renisus. 2009. Available online: http://www.saude.gov.br/acoes-e-programas/programa-nacional-de-plantas-medicinais-e-fitoterapicos-ppnpmf/politica-e-programa-nacional-de-plantas-medicinais-e-fitoterapicos/plantas-medicinais-de-interesse-ao-sus-renisus (accessed on 14 August 2019).
- Penido, A.B.; de Morais, S.M.; Ribeiro, A.B.; Silva, A.Z. Ethnobotanical study of medicinal plants in Imperatriz, State of Maranhão, Northeastern Brazil. Acta Amazon. 2016, 46, 345–354. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Castro, J.A.M.; Monteiro, O.S.; Coutinho, D.F.; Rodrigues, A.A.C.; da Silva, J.K.R.; Maia, J.G.S. Seasonal and circadian study of a thymol/γ-terpinene/p-cymene type oil of Ocimum gratissimum L. and Its antioxidant and antifungal effects. J. Braz. Chem. Soc. 2019, 30, 930–938. [Google Scholar] [CrossRef]
- Iwalokun, B.A.; Gbenle, G.O.; Adewole, T.A.; Smith, S.I.; Akinsinde, K.A.; Omonigbehin, E.O. Effects of Ocimum gratissimum L essential oil at subinhibitory concentrations on virulent and multidrug-resistant Shigella strains from Lagos, Nigeria. Apmis 2003, 111, 477–482. [Google Scholar] [CrossRef]
- Silva, N.C.C.; Fernandes Júnior, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Intorasoot, A.; Chornchoem, P.; Sookkhee, S.; Intorasoot, S. Bactericidal activity of herbal volatile oil extracts against multidrug-resistant Acinetobacter baumannii. J. Ethnopharmacol. 2017, 6, 218–222. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Faria, T.J.; Ferreira, R.S.; Yassumoto, L.; de Souza, J.R.P.; Ishikawa, N.K.; Barbosa, A.M. Antifungal activity of essential oil isolated from Ocimum gratissimum L. (eugenol chemotype) against phytopathogenic fungi. Braz. Arch. Biol. Technol. 2006, 49, 867–871. [Google Scholar] [CrossRef]
- Ocheng, F.; Bwanga, F.; Joloba, M.; Softrata, A.; Azeem, M.; Pütsep, K.; Borg-Karlson, A.K.; Obua, C.; Gustafsson, A. Essential oils from Ugandan aromatic medicinal plants: Chemical composition and growth inhibitory effects on oral pathogens. Evid. Based Complement. Alternat. Med. 2015, 2015, 230832. [Google Scholar] [CrossRef] [PubMed]
- Matasyoha, L.G.; Matasyoh, J.C.; Wachira, F.N.; Kinyua, M.G.; Muigai, A.W.T.; Mukiama, T.K. Antimicrobial activity of essential oils of Ocimum gratissimum L. from different populations of Kenya. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 187–193. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical composition, in vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Indian J. Pharm. Sci. 2013, 75, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Dhar, T.N.; Sengupta, C.; Ghosh, P. Biological activities of essential oils and methanol extracts of five Ocimum species against pathogenic bacteria. Czech J. Food Sci. 2013, 31, 194–202. [Google Scholar] [CrossRef]
- Tangpao, T.; Chung, H.H.; Sommano, S.R. Aromatic profiles of essential oils from five commonly used Thai Basils. Foods 2018, 7, 175. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Hwang, D.; Park, S.M.; Kim, B.; Lee, H.M. Chemical composition, antioxidant and anti-melanogenic activities of essential oils from Chrysanthemum boreale MAKINO at different harvesting stages. Chem. Biodivers. 2018, 15, e1700506. [Google Scholar] [CrossRef]
- Kpadonou Kpoviessi, B.G.H.; Ladekana, E.Y.; Kpoviessia, D.S.S.; Gbaguidib, F.; Yehouenoud, B.; Quetin-Leclercq, J.; Figueredoe, G.; Moudachiroub, M.; Accrombessi, G.C. Chemical variation of essential oil constituents of Ocimum gratissimum L. from Benin, and impact on antimicrobial properties and toxicity against Artemia salina LEACH. Chem. Biodivers. 2012, 9, 139–150. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P.L. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Yamani, H.A.; Pang, E.C.; Mantri, N.; Deighton, M.A. Antimicrobial activity of Tulsi (Ocimum tenuiflorum) essential oil and their major constituents against three species of bacteria. Front. Microbiol. 2016, 7, 681. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Ag. Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Gao, J.P.; Xia, J.L.; Ritenour, M.A.; Li, G.Y.; Shan, Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. LWT 2018, 97, 825–839. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.; Dal Maso, S.; Foncoux, B.; Kamili, A.; Laurin, Y.; Genva, M.; Jijakli, H.M.; De Clerck, C.; Fauconnier, M.L.; Deleu, M. Insights into the relationships between herbicide activities, molecular structure and membrane interaction of cinnamon and citronella essential oils components. Int. J. Mol. Sci. 2019, 20, 4007. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Kong, L.C.; Liu, J.; Ma, H.X. Synergistic effect of eugenol with colistin against clinical isolated colistin-resistant Escherichia coli strains. Antimicrob. Resist. Infect. Control. 2018, 7, 17. [Google Scholar] [CrossRef]
- Chimnoi, N.; Reuk-ngam, N.; Chuysinuan, P.; Khlaychan, P.; Khunnawutmanotham, N.; Chokchaichamnankit, D.; Thamniyom, W.; Klayraung, S.; Mahidol, C.; Techasakul, S. Characterization of essential oil from Ocimum gratissimum leaves: Antibacterial and mode of action against selected gastroenteritis pathogens. Microb. Pathogenesis. 2018, 118, 290–300. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Palaniappan, K.; Holley, R.A. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food. Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Singh, B.R.; Sinha, D.K.; Kumar, V.; Prasanna Vadhana, O.R.; Varan Singh, S.; Nirupama, K.R.; Pruthvishree; Archana Saraf, B.S. Potential of herbal drug and antibiotic combination therapy: A new approach to treat multidrug resistant bacteria. Pharm. Anal. Acta 2016, 7, 1000523. [Google Scholar] [CrossRef]
- Gallucci, N.; Casero, C.; Oliva, M.; Zygadlo, J.; Demo, M. Interaction between terpenes and penicillin on bacterial strains resistant to betalactam antibiotics. Mol. Med. Chem. 2006, 10, 30–32. [Google Scholar]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.D.L.; Rodrigue, F.F.G.; Coutinho, H.D.M.; da Costa, J.G.M.; de Menezes, I.R.A. Chemical composition, modulatory bacterial resistance and antimicrobial activity of essential oil the Hyptis martiusii benth. by direct and gaseous contact. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e13521. [Google Scholar] [CrossRef]
- Oliva, A.; Costantini, S.; de Angelis, M.; Garzoli, S.; Božović, M.; Mascellino, M.T.; Vullo, V.; Ragno, R. High potency of Melaleuca alternifolia essential oil against multi-drug resistant gram-negative bacteria and methicillin-resistant Staphylococcus aureus. Molecules 2018, 23, 2584. [Google Scholar] [CrossRef]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Chaabouni, Y.; Mahdouani, K.; Bakhrouf, A.; Chaieb, K. Use of carvacrol, thymol, and eugenol for biofilm eradication and resistance modifying susceptibility of Salmonella enterica serovar Typhimurium strains to nalidixic acid. Microb. Pathog. 2017, 104, 56–63. [Google Scholar] [CrossRef]
- Fadli, M.; Saada, A.; Sayadi, S.; Chevalier, J.; Mezrioui, N.E.; Pagès, J.M.; Hassani, L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection – bacteria and their synergistic potential with antibiotics. Phytomedicine 2012, 19, 464–471. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Rao, H.; Lai, P.; Gao, Y. Chemical composition, antibacterial activity, and synergistic effects with conventional antibiotics and nitric oxide production inhibitory activity of essential oil from Geophila repens (L.) I.M. Johnst. Molecules 2017, 22, 1561. [Google Scholar] [CrossRef]
- Aelenei, P.; Rimbu, C.M.; Guguianu, E.; Dimitriu, G.; Aprotosoaie, A.C.; Brebu, M.; Horhogea, C.E.; Miron, A. Coriander essential oil and linalool–interactions with antibiotics against Gram-positive and Gram-negative bacteria. Lett. Appl. Microbiol. 2019, 68, 156–164. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Mai, C.W.; Lim, W.M.; Yap, W.S.; Lim, S.H.E.; Lai, K.S. Additivity vs. synergism: Investigation of the additive interaction of cinnamon bark oil and meropenem in combinatory therapy. Molecules 2017, 22, 1733. [Google Scholar] [CrossRef] [PubMed]
- Rubini, D.; Banu, S.F.; Nisha, P.; Murugan, R.; Thamotharan, S.; Percino, M.J.; Subramani, P.; Nithyanand, P. Essential oils from unexplored aromatic plants quench biofilm formation and virulence of Methicillin resistant Staphylococcus aureus. Microb. Pathog. 2018, 122, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlind, R.P.; Hall-Stoodleye, L.; Stoodley, P. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, V.A.; Santos, H.S.; Arruda, F.V.S.; Bandeira, P.N.; Albuquerque, M.R.J.R.; Pereira, M.O.; Henriques, M.; Cavada, B.S.; Teixeira, E.H. Casbane diterpene as a promising natural antimicrobial agent against biofilm-associated infections. Molecules 2011, 16, 190–201. [Google Scholar] [CrossRef]
- Budzyńska, A.; Rożalska, S.; Sadowska, B.; Rożalska, B. Candida albicans/Staphylococcus aureus dual-species biofilm as a target for the combination of essential oils and fluconazole or mupirocin. Mycopathologia 2017, 182, 989–995. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Gwon, G.; Kim, S.I.; Park, J.G.; Lee, J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 36377. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biolms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, F.S.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Rathinam, P.; Viswanathan, P. Anti-virulence potential of eugenol-rich fraction of Syzygium aromaticum against multidrug resistant uropathogens isolated from catheterized patients. Avicenna J. Phytomed. 2018, 8, 416–431. [Google Scholar]
- Ghosh, T.; Das, A.B.; Jena, B.; Pradhan, C. Antimicrobial effect of silver zinc oxide (Ag-ZnO) nanocomposite particles. Front. Life Sci. 2014, 8, 47–54. [Google Scholar] [CrossRef]
- Mojsoska, B.; Carretero, G.; Larsen, S.; Mateiu, R.V.; Jenssen, H. Peptoids successfully inhibit the growth of gram negative E-coli causing substantial membrane damage. Sci. Rep. 2017, 14, 42332. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Ahmad, I.; Khan, M.S.; Ahmad, E.; Tahseen, Q.; Khan, M.S.; Alshabib, N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, J.P.; Guinoiseau, E.; Serra, D.R.; Sutour, S.; Paoli, M.; Tomi, F.; Quilichini, Y.; Berti, L.; Lorenzi, V. Anti-quorum sensing activity of 12 essential oils on Chromobacterium violaceum and specific action of cis-cis-p-menthenolide from Corsican Mentha suaveolens ssp. Insularis. Molecules 2018, 23, 2125. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou1, Y.; Cheng, Q.; Zhu1, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Dool, H.D.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Almeida, M.V.A.; Cangussú, Í.M.; Carvalho, A.L.S.; Brito, I.L.P.; Costa, R.A. Drug resistance, AmpC-β-lactamase and extended-spectrum β-lactamase-producing Enterobacteriaceae isolated from fish and shrimp. Rev. Inst. Med. Trop. São Paulo. 2017, 59, e70. [Google Scholar] [CrossRef]
- CLS. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard, 10th ed.; CLSI document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Field, D.; Quigley, L.; O’Connor, P.M.; Rea, M.C.; Daly, K.; Cotter, P.D.; Hill, C.; Ross, R.P. Studies with bioengineered Nisin peptides highlight the broad-spectrum potency of Nisin V. Microb. Biotechnol. 2010, 3, 473–486. [Google Scholar] [CrossRef] [Green Version]
- White, R.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrob. Ag. Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases. Terminology Relating to Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents; EUCAST Definitive Document E. Def 1.2; European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID): Basel, Switzerland, 2000. [Google Scholar]
Sample Availability: Samples of the compounds EOOG, Ocimum gratissimum EO, are available from the authors. |





| Peak | Compounds a | Chemical Class | KIL b | KIC c | % |
|---|---|---|---|---|---|
| 1 | α-Pinene | HM d | 939 | 943 | 0.08 |
| 2 | Sabinene | HM | 975 | 982 | 0.17 |
| 3 | β-Pinene | HM | 979 | 986 | 0.43 |
| 4 | Myrcene | HM | 990 | 995 | 0.14 |
| 5 | 1,8-Cineole | OM e | 1031 | 1040 | 15.16 |
| 6 | (E)-β-Ocimene | HM | 1050 | 1053 | 0.10 |
| 7 | Linalool | OM | 1096 | 1103 | 0.34 |
| 8 | δ-Terpineol | OM | 1166 | 1174 | 0.12 |
| 9 | Terpinen-4-ol | OM | 1177 | 1184 | 0.16 |
| 10 | α-Terpineol | OM | 1188 | 1196 | 0.31 |
| 11 | Eugenol | PH f | 1359 | 1365 | 74.83 |
| 12 | (E)-Caryophyllene | HS g | 1419 | 1427 | 2.20 |
| 13 | α-Humulene | HS | 1454 | 1461 | 0.32 |
| 14 | γ-Muurolene | HS | 1479 | 1488 | 0.51 |
| 15 | β-Selinene | HS | 1490 | 1493 | 2.82 |
| 16 | α-Selinene | HS | 1498 | 1501 | 0.85 |
| 17 | 7-Epi-α-selinene | HS | 1522 | 1525 | 0.26 |
| 18 | Spathulenol | OS h | 1578 | 1584 | 0.07 |
| 19 | Caryophyllene oxide | OS | 1583 | 1590 | 0.55 |
| Total | 99.42 | ||||
| Staphylococcus aureus | IZD (mm) 1 | MIC (µg/mL) 2 | MBC (µg/mL) 3 |
| ATCC 6538 | 17 | 1000 | 1000 |
| 2B | 14 | 1000 | 2000 |
| 5B | 20 | 2000 | 2000 |
| 7B | 15 | 1000 | 2000 |
| Escherichia coli | |||
| ATCC 11303 | 12 | 1000 | 1000 |
| P12 | 13 | 1000 | 1000 |
| P25 | 12 | 1000 | 1000 |
| P36 | 13 | 1000 | 1000 |
| Microorganisms | Combination | MIC (µg/mL) | FICi | Interpretation | Drug Reduction | |
|---|---|---|---|---|---|---|
| Individual | Combined | |||||
| S. aureus (5B) | EOOG | 2000 | 1000 | 0.516 | Additive | 2x |
| OXA | 2000 | 31.25 | 64x | |||
| EOOG | 2000 | 1000 | 0.562 | Additive | 2x | |
| CIP | 62.50 | 3.90 | 16x | |||
| E. coli (P12) | EOOG | 1000 | 1000 | 2.000 | Antagonistic | NR |
| CIP | 62.50 | 62.50 | NR | |||
| Staphylococcus aureus | Source | Antibiotic Resistance |
| Standard | ATCC 6538 | Sensitive |
| 2B | Soft tissues | ERT, CLIN e BZP |
| 5B | Human blood | ERT, CLIN, CIP, NOR, MOX, BZP, OXA e RIP (I) |
| 7B | Human blood | BZP e OXA |
| Escherichia coli | ||
| Standard | ATCC 11303 | Sensitive |
| P12 | Fish fillet | AMP, CFL, CIP, NOR, NAL e AMC (I) |
| P25 | Fish fillet | AMP, CFL (I) e AMC (I) |
| P36 | Fish fillet | AMP, CFL (I) e AMC (I) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, R.S.; Albuquerque Azevedo, Á.M.; Gomes Pereira, A.M.; Rocha, R.R.; Bastos Cavalcante, R.M.; Carneiro Matos, M.N.; Ribeiro Lopes, P.H.; Gomes, G.A.; Soares Rodrigues, T.H.; Santos, H.S.d.; et al. Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli. Molecules 2019, 24, 3864. https://doi.org/10.3390/molecules24213864
Melo RS, Albuquerque Azevedo ÁM, Gomes Pereira AM, Rocha RR, Bastos Cavalcante RM, Carneiro Matos MN, Ribeiro Lopes PH, Gomes GA, Soares Rodrigues TH, Santos HSd, et al. Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli. Molecules. 2019; 24(21):3864. https://doi.org/10.3390/molecules24213864
Chicago/Turabian StyleMelo, Ramaiana Soares, Águida Maria Albuquerque Azevedo, Antônio Mateus Gomes Pereira, Renan Rhonalty Rocha, Rafaela Mesquita Bastos Cavalcante, Maria Nágila Carneiro Matos, Pedro Henrique Ribeiro Lopes, Geovany Amorim Gomes, Tigressa Helena Soares Rodrigues, Hélcio Silva dos Santos, and et al. 2019. "Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli" Molecules 24, no. 21: 3864. https://doi.org/10.3390/molecules24213864
APA StyleMelo, R. S., Albuquerque Azevedo, Á. M., Gomes Pereira, A. M., Rocha, R. R., Bastos Cavalcante, R. M., Carneiro Matos, M. N., Ribeiro Lopes, P. H., Gomes, G. A., Soares Rodrigues, T. H., Santos, H. S. d., Ponte, I. L., Costa, R. A., Brito, G. S., Catunda Júnior, F. E. A., & Carneiro, V. A. (2019). Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli. Molecules, 24(21), 3864. https://doi.org/10.3390/molecules24213864