Mannosylated Polyrotaxanes for Increasing Cellular Uptake Efficiency in Macrophages through Receptor-Mediated Endocytosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Man-PRX
2.2. Analysis of the Binding of Man-PRX to the MMR
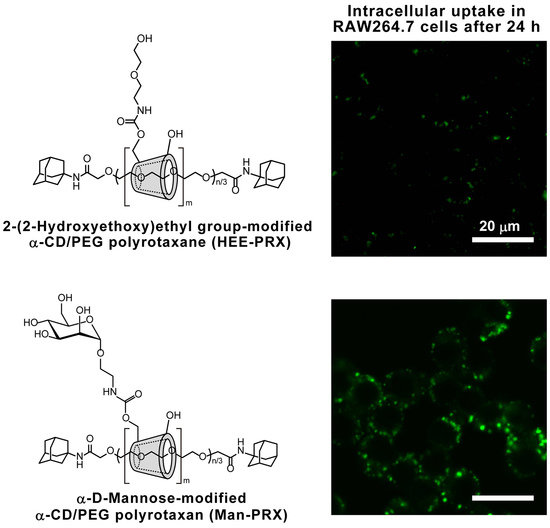
2.3. Intracellular Man-PRX Uptake in RAW264.7 Cells
2.4. Effect of RAW264.7 Cell Polarization on the Intracellular Man-PRX Uptake
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of Man-PRX
3.4. Synthesis of BODIPY-Labeled PRX
3.5. Analysis of the Binding between Man-PRX and the MMR by SPR
3.6. Cell Culture
3.7. Expression Level of the MMR
3.8. Flow Cytometry Analysis of Intracellular Man-PRX Uptake
3.9. Inhibition of Intracellular Man-PRX Uptake
3.10. CLSM Observation
3.11. Effect of RAW264.7 Cell Polarization on the Intracellular Man-PRX Uptake
3.12. Quantitative RT-PCR
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PRX | Polyrotaxane |
| CD | Cyclodextrin |
| MMR | Macrophage mannose receptor |
| Man-PRX | α-d-Mannose-modified polyrotaxane |
| HEE-PRX | 2-(2-Hydroxyethoxy)ethyl carbamate-modified polyrotaxane |
| PEG | Poly(ethylene glycol) |
| CDI | 1,1′-Carbonyldiimidazole |
| DMSO | Dimethyl sulfoxide |
| Man-NH2 | 2-Aminoethyl α-d-mannopyranoside |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| SEC | Size exclusion chromatography |
| FT-IR | Fourier transform infrared |
| NMR | Nuclear magnetic resonance |
| SPR | Surface plasmon resonance |
| APC | Allophycocyanin |
| CLSM | Confocal laser scanning microscopy |
| LPS | Lipopolysaccharide |
| IL | Interleukin |
| IFN-γ | Interferon γ |
| TNF-α | Tumor necrosis factor α |
| Arg-1 | Arginase-1 |
| MWCO | Molecular weight cut-off |
| HEPES | 2-[4-(2-Hydroxyethyl)piperazin-1-yl]ethanesulfonic acid |
| DMEM | Dulbecco’s modified Eagle’s medium. |
References
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: A rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Wenz, G.; Han, B.; Müller, A. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Ito, K. Recent advances in the preparation of cyclodextrin-based polyrotaxanes and their applications to soft materials. Soft Matter 2007, 3, 1456–1473. [Google Scholar] [CrossRef]
- Tamura, A.; Yui, N. Threaded macromolecules as a versatile framework for biomaterials. Chem. Commun. 2014, 50, 13433–13446. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Arisaka, Y.; Yui, N. Emergence of intelligent functions with supramolecular polymers and their biomaterials applications. Kobunshi Ronbunshu 2017, 74, 239–249. [Google Scholar] [CrossRef]
- Tamura, A.; Yui, N. Lysosomal-specific cholesterol reduction by biocleavable polyrotaxanes for ameliorating Niemann-Pick type C disease. Sci. Rep. 2014, 4, 4356. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Yui, N. β-Cyclodextrin-threaded biocleavable polyrotaxanes ameliorate impaired autophagic flux in Niemann-Pick type C disease. J. Biol. Chem. 2015, 290, 9441–9454. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Yui, N. Polyrotaxane-based systemic delivery of β-cyclodextrins for potentiating therapeutic efficacy in a mouse model of Niemann-Pick type C disease. J. Control. Release 2018, 269, 148–158. [Google Scholar] [CrossRef]
- Tamura, A.; Ohashi, M.; Nishida, K.; Yui, N. Acid-induced intracellular dissociation of β-cyclodextrin-threaded polyrotaxanes directed toward attenuating phototoxicity of bisretinoids through promoting excretion. Mol. Pharmaceut. 2017, 14, 4714–4724. [Google Scholar] [CrossRef]
- Nishida, K.; Tamura, A.; Yui, N. ER stress-mediated autophagic cell death induction through methylated β-cyclodextrins-threaded acid-labile polyrotaxanes. J. Control. Release 2018, 275, 20–31. [Google Scholar] [CrossRef]
- Tamura, A.; Yui, N. Rational design of stimuli-cleavable polyrotaxanes for therapeutic applications. Polym. J. 2017, 49, 527–534. [Google Scholar] [CrossRef]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Gordon, S.; Martinez-Pomares, L. The mannose receptor: Linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005, 26, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pomares, L. The mannose receptor. J. Leukocyte Biol. 2012, 92, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.; Schlesinger, P.H.; Sigardson, E.; Rodman, J.S.; Lee, Y.C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: Characterization and evidence for receptor recycling. Cell 1980, 19, 207–215. [Google Scholar] [CrossRef]
- Irache, J.M.; Salman, H.H.; Gamazo, C.; Espuelas, S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin. Drug Deliv. 2008, 5, 703–724. [Google Scholar] [CrossRef]
- Vedove, E.D.; Costabile, G.; Merkel, O.M. Mannose and mannose-6-phosphate receptor-targeted drug delivery systems and their application in cancer therapy. Adv. Healthc. Mater. 2018, 7, 1701398. [Google Scholar] [CrossRef]
- Fujita, T.; Nishikawa, M.; Tamaki, C.; Takakura, Y.; Hashida, M.; Sezaki, H. Targeted delivery of human recombinant superoxide dismutase by chemical modification with mono- and polysaccharide derivatives. J. Pharmacol. Exp. Ther. 1992, 263, 971–978. [Google Scholar] [PubMed]
- Kawakami, S.; Sato, A.; Nishikawa, M.; Yamashita, F.; Hashida, M. Mannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomes. Gene Ther. 2000, 7, 292–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahara, N.; Mukherjee, J.; de Haas, H.J.; Petrov, A.D.; Tawakol, A.; Haider, N.; Tahara, A.; Constantinescu, C.C.; Zhou, J.; Boersma, H.H.; et al. 2-deoxy-2-[18F]fluoro-d-mannose positron emission tomography imaging in atherosclerosis. Nat. Med. 2014, 20, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Rajaram, M.V.S.; Schlesinger, L.S. Exploitation of the macrophage mannose receptor (CD206) in infectious disease diagnostics and therapeutics. J. Cytol. Mol. Biol. 2014, 1, 1000003. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Hyun, H.; Bao, K.; Lee, J.H.; El Fakhri, G.; Choi, Y.; Choi, H.S. Multivalent mannose-decorated NIR nanoprobes for targeting pan lymph nodes. Chem. Eng. J. 2018, 340, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ehashi, T.; Hyun, H.; Yui, N. Anti-inflammatory response of mannose-conjugated polyrotaxane endocytosed into macrophage. Macromol. Res. 2011, 19, 495–500. [Google Scholar] [CrossRef]
- Hyun, H.; Yui, N. Mono-, di-, or triazidated cyclodextrin-based polyrotaxanes for facile and efficient functionalization via click chemistry. Macromol. Rapid Commun. 2011, 32, 326–331. [Google Scholar] [CrossRef]
- Hyun, H.; Yui, N. Ligand accessibility to receptor binding sites enhanced by movable polyrotaxanes. Macromol. Biosci. 2011, 11, 765–771. [Google Scholar] [CrossRef]
- Hu, X.; Gao, J.; Luo, Y.; Wei, T.; Dong, Y.; Chen, G.; Chen, H. One-pot multicomponent synthesis of glycopolymers through a combination of host–guest interaction, thiol-ene, and copper-catalyzed click reaction in water. Macromol. Rapid Commun. 2017, 38, 1700434. [Google Scholar] [CrossRef]
- Tamura, A.; Nishida, K.; Yui, N. Lysosomal pH-inducible supramolecular dissociation of polyrotaxanes possessing acid-labile N-triphenylmethyl end groups and their therapeutic potential for Niemann-Pick type C disease. Sci. Technol. Adv. Mater. 2016, 17, 361–374. [Google Scholar] [CrossRef]
- Tamura, A.; Ohashi, M.; Yui, N. Oligo(ethylene glycol)-modified β-cyclodextrin-based polyrotaxanes for simultaneously modulating solubility and cellular internalization efficiency. J. Biomater. Sci. Polym. Edit. 2017, 28, 1124–1139. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Song, B.; He, P.; Wang, Z.; Wang, J. Electrochemical impedance spectroscopy (EIS) study on the degradation of acrylic polyurethane coatings. RSC Adv. 2017, 7, 13742–13748. [Google Scholar] [CrossRef] [Green Version]
- Harada, A.; Li, J.; Kamachi, M. Formation of inclusion complexes of monodisperse oligo(ethy1ene glycol)s with α-cyclodextrin. Macromolecules 1994, 27, 4538–4543. [Google Scholar] [CrossRef]
- Ruan, G.X.; Chen, Y.Z.; Yao, X.L.; Du, A.; Tang, G.P.; Shen, Y.Q.; Tabata, Y.; Gao, J.Q. Macrophage mannose receptor-specific gene delivery vehicle for macrophage engineering. Acta Biomater. 2014, 10, 1847–1855. [Google Scholar] [CrossRef]
- Martinez-Pomares, L.; Reid, D.M.; Brown, G.D.; Taylor, P.R.; Stillion, R.J.; Linehan, S.A.; Zamze, S.; Gordon, S.; Wong, S.Y. Analysis of mannose receptor regulation by IL-4, IL-10, and proteolytic processing using novel monoclonal antibodies. J. Leukoc. Biol. 2003, 73, 604–613. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Edit. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; de Dios Ruiz-Rosado, J.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.; Ahmed, M.; et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Roura, S.; Gálvez-Montón, C.; Pujal, J.M.; Aran, G.; Sanjurjo, L.; Franquesa, M.; Sarrias, M.; Bayes-Genis, A.; Borràs, F.E. Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells: Implications for nanomedicine. Theranostics 2017, 7, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Zhao, C.; Ito, K. Efficient production of polyrotaxanes from α-cyclodextrin and poly (ethylene glycol). Macromolecules 2005, 38, 7524–7527. [Google Scholar] [CrossRef]
- Sardzík, R.; Noble, G.T.; Weissenborn, M.J.; Martin, A.; Webb, S.J.; Flitsch, S.L. Preparation of aminoethyl glycosides for glycoconjugation. Beilstein J. Org. Chem. 2010, 6, 699–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, E.S.; Lee, G.; Lee, C.; Ye, M.; Chung, H.S.; Kim, H.; Bae, S.J.; Hwang, D.S.; Bae, H. Bee venom phospholipase A2, a novel Foxp3+ regulatory T cell inducer, protects dopaminergic neurons by modulating neuroinflammatory responses in a mouse model of Parkinson’s disease. J. Immunol. 2015, 195, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Frison, N.; Taylor, M.E.; Soilleux, E.; Bousser, M.T.; Mayer, R.; Monsigny, M.; Drickamer, K.; Roche, A.C. Oligolysine-based oligosaccharide clusters: Selective recognition and endocytosis by the mannose receptor and dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin. J. Biol. Chem. 2003, 278, 23922–23929. [Google Scholar] [CrossRef] [PubMed]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibaguchi, K.; Tamura, A.; Terauchi, M.; Matsumura, M.; Miura, H.; Yui, N. Mannosylated Polyrotaxanes for Increasing Cellular Uptake Efficiency in Macrophages through Receptor-Mediated Endocytosis. Molecules 2019, 24, 439. https://doi.org/10.3390/molecules24030439
Shibaguchi K, Tamura A, Terauchi M, Matsumura M, Miura H, Yui N. Mannosylated Polyrotaxanes for Increasing Cellular Uptake Efficiency in Macrophages through Receptor-Mediated Endocytosis. Molecules. 2019; 24(3):439. https://doi.org/10.3390/molecules24030439
Chicago/Turabian StyleShibaguchi, Kai, Atsushi Tamura, Masahiko Terauchi, Mitsuaki Matsumura, Hiroyuki Miura, and Nobuhiko Yui. 2019. "Mannosylated Polyrotaxanes for Increasing Cellular Uptake Efficiency in Macrophages through Receptor-Mediated Endocytosis" Molecules 24, no. 3: 439. https://doi.org/10.3390/molecules24030439
APA StyleShibaguchi, K., Tamura, A., Terauchi, M., Matsumura, M., Miura, H., & Yui, N. (2019). Mannosylated Polyrotaxanes for Increasing Cellular Uptake Efficiency in Macrophages through Receptor-Mediated Endocytosis. Molecules, 24(3), 439. https://doi.org/10.3390/molecules24030439