Three New 2-(2-Phenylethyl)chromone Derivatives of Agarwood Originated from Gyrinops salicifolia
Abstract
:1. Introduction
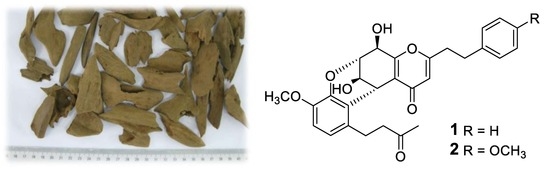
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Bioassays
3.4.1. Bioassay for AChE Inhibitory Activity In Vitro
3.4.2. Bioassay for Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borris, R.P.; Blaskó, G.; Cordell, G.A. Ethnopharmacologic and phytochemical studies of the Thymelaeaceae. J. Ethnopharmacol. 1988, 24, 41–91. [Google Scholar] [CrossRef]
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour. Fragr. J. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Mei, W.L.; Zeng, Y.B.; Guo, Z.K.; Zhao, Y.X.; Wang, H.; Zuo, W.J.; Dong, W.H.; Wang, Q.H.; Dai, H.F. 2-(2-phenylethyl)chromone derivatives in Chinese Agarwood “Qi-Nan” from Aquilaria sinensis. Planta Med. 2013, 79, 1329–1334. [Google Scholar]
- Li, W.; Cai, C.H.; Dong, W.H.; Guo, Z.K.; Wang, H.; Mei, W.L.; Dai, H.F. 2-(2-Phenylethyl)chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapia 2014, 98, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A. Natural occurring 2-(2-phenylethyl) chromones, structure elucidation and biological activities. Nat. Prod. Res. 2015, 29, 1489–1520. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Wang, H.; Guo, Z.K.; Li, W.; Mei, W.L.; Dai, H.F. Fragrant agarofuran and eremophilane sesquiterpenes in agarwood “Qi-Nan” from Aquilaria sinensis. Phytochem. Lett. 2014, 8, 121–125. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.H.; Guo, Z.K.; Wang, H.; Zuo, W.J.; Dong, W.H.; Mei, W.L.; Dai, H.F. Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia 2015, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Mei, W.L.; Dong, W.H.; Gai, C.J.; Li, W.; Zhu, G.P.; Dai, H.F. 2-(2-Phenylethyl) chromone Derivatives of Agarwood Originating from Gyrinops salicifolia. Molecules 2016, 21, 1313. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Mei, W.L.; Kong, F.D.; Dong, W.H.; Gai, C.J.; Li, W.; Zhu, G.P.; Dai, H.F. Sesquiterpenes of agarwood from Gyrinops salicifolia. Fitoterapia 2016, 113, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.X.; Zhu, Z.X.; Song, Y.L.; Shi, S.P.; Sun, H.; Zhao, Y.F.; Zheng, J.; Ferreira, D.; Zjawiony, J.K.; Tu, P.F.; Li, J. Anti-inflammatory dimeric 2-(2-phenylethyl) chromones from the resinous wood of Aquilaria sinensis. J. Nat. Prod. 2017, 81, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Mei, W.L.; Kong, F.D.; Li, W.; Yuan, J.Z.; Dai, H.F. 5,6,7,8-Tetrahydro-2-(2-phenylethyl) chromones from artificial agarwood of Aquilaria sinensis and their inhibitory activity against acetylcholinesterase. Phytochemistry 2017, 139, 98–108. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–3 are available from the authors. |


| Position | 1 a | Unit A of 4 b | 2 c | Unit A of 5 d | 3 e |
|---|---|---|---|---|---|
| 3 | 6.07, s | 6.42, s | 6.05, s | 6.02, s | 6.22, s |
| 5 | 4.31, t (2.1) | 4.29, t (2.5) | 4.53, dd (3.0, 1.9) | 4.49, br s | |
| 6 | 4.23, dd (3.4, 2.1) | 4.40, m | 4.41, dd (4.9, 3.0) | 4.32, dd (4.5, 3.0) | 6.79, d (8.3) |
| 7 | 4.61, m | 4.87, br s | 4.74, dt (4.9, 1.9) | 4.73, m | 7.52, t (8.3) |
| 8 | 4.33, br s | 4.55, d (7.0) | 4.47, d (1.9) | 4.46, d (2.0) | 6.96, d (8.3) |
| 2′ | 7.18, d (7.2) | 7.27, m | 7.07, d (8.6) | 7.06, d (8.5) | 7.20, d (2.1) |
| 3′ | 7.22, t (7.2) | 7.27, m | 6.76, d (8.6) | 6.75, d (8.5) | |
| 4′ | 7.14, t (7.2) | 7.19, m | |||
| 5′ | 7.22, t (7.2) | 7.27, m | 6.76, d (8.6) | 6.75, d (8.5) | 6.89, d (8.3) |
| 6′ | 7.18, d (7.2) | 7.27, m | 7.07, d (8.6) | 7.06, d (8.5) | 7.10, dd (8.3, 2.1) |
| 7′ | 2.81, 2.89, m | 2.99, m | 2.84, 2.93, m | 2.90, m | 7.54, d (15.9) |
| 8′ | 2.81, m | 2.92, m | 2.93, m | 2.82, m | 6.61, d (15.9) |
| 5′′ | 6.70, d (8.3) | 6.76, d (8.3) | |||
| 6′′ | 6.56, d (8.3) | 6.66, d (8.3) | |||
| 7′′ | 2.76, 3.48, m | 2.93, 3.64, m | |||
| 8′′ | 2.48, 2.61, m | 2.61, 2.71, m | |||
| 10′′ | 2.06, s | 2.18, s | |||
| 4′-OCH3 | 3.73, s | 3.95, s | |||
| 4″-OCH3 | 3.64, s | 3.77, s | |||
| 6-OH | 5.87, d (3.0) | ||||
| 8-OH | 6.11, d (8.0) |
| Position | 1 a | Unit A of 4 b | 2 c | Unit A of 5 d | 3 e |
|---|---|---|---|---|---|
| 2 | 168.0, C | 170.1, C | 170.7, C | 170.6, C | 163.3, C |
| 3 | 112.7, CH | 111.8, CH | 113.8, CH | 113.7, CH | 108.5, CH |
| 4 | 178.0, C | 180.0, C | 181.0, C | 180.9, C | 183.6, C |
| 5 | 31.7, CH | 29.3, CH | 33.4, CH | 33.4, CH | 160.9, C |
| 6 | 63.6, CH | 61.3, CH | 65.6, CH | 65.5, CH | 111.3, CH |
| 7 | 74.7, CH | 77.2, CH | 75.6, CH | 75.8, CH | 135.3, CH |
| 8 | 68.8, CH | 68.1, CH | 70.4, CH | 70.3, CH | 106.9, CH |
| 9 | 162.5, C | 164.1, C | 164.3, C | 164.3, C | 156.3, C |
| 10 | 121.7, C | 121.1, C | 122.9, C | 122.8, C | 111.0, C |
| 1′ | 140.3, C | 140.0, C | 133.0, C | 133.1, C | 128.6, C |
| 2′ | 128.6, CH | 128.3, CH | 130.4, CH | 130.4, CH | 112.8, CH |
| 3′ | 128.7, CH | 128.4, CH | 114.9, CH | 114.9, CH | 146.1, C |
| 4′ | 126.5, CH | 126.2, CH | 159.7, C | 159.7, C | 148.5, C |
| 5′ | 128.7, CH | 128.4, CH | 114.9, CH | 114.9, CH | 110.8, CH |
| 6′ | 128.6, CH | 128.3, CH | 130.4, CH | 130.4, CH | 121.7, CH |
| 7′ | 32.2, CH2 | 32.1, CH2 | 33.1, CH2 | 33.1, CH2 | 138.0, CH |
| 8′ | 34.3, CH2 | 34.6, CH2 | 36.5, CH2 | 36.5, CH2 | 117.9, CH |
| 1′′ | 131.6, C | 133.2, C | |||
| 2′′ | 123.0, C | 123.9, C | |||
| 3′′ | 141.3, C | 142.8, C | |||
| 4′′ | 146.1, C | 147.6, C | |||
| 5′′ | 111.1, CH | 112.4, CH | |||
| 6′′ | 120.9, CH | 122.3, CH | |||
| 7′′ | 25.6, CH2 | 26.9, CH2 | |||
| 8′′ | 45.4, CH2 | 46.6, CH2 | |||
| 9′′ | 208.7, C | 211.6, C | |||
| 10′′ | 30.0, CH3 | 30.0, CH3 | |||
| 4′-OCH3 | 55.6, CH3 | 56.1, CH3 | |||
| 4″-OCH3 | 55.6, CH3 | 56.5, CH3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.-H.; Wang, H.; Guo, F.-J.; Mei, W.-L.; Chen, H.-Q.; Kong, F.-D.; Li, W.; Zhou, K.-B.; Dai, H.-F. Three New 2-(2-Phenylethyl)chromone Derivatives of Agarwood Originated from Gyrinops salicifolia. Molecules 2019, 24, 576. https://doi.org/10.3390/molecules24030576
Dong W-H, Wang H, Guo F-J, Mei W-L, Chen H-Q, Kong F-D, Li W, Zhou K-B, Dai H-F. Three New 2-(2-Phenylethyl)chromone Derivatives of Agarwood Originated from Gyrinops salicifolia. Molecules. 2019; 24(3):576. https://doi.org/10.3390/molecules24030576
Chicago/Turabian StyleDong, Wen-Hua, Hao Wang, Feng-Juan Guo, Wen-Li Mei, Hui-Qin Chen, Fan-Dong Kong, Wei Li, Kai-Bing Zhou, and Hao-Fu Dai. 2019. "Three New 2-(2-Phenylethyl)chromone Derivatives of Agarwood Originated from Gyrinops salicifolia" Molecules 24, no. 3: 576. https://doi.org/10.3390/molecules24030576
APA StyleDong, W. -H., Wang, H., Guo, F. -J., Mei, W. -L., Chen, H. -Q., Kong, F. -D., Li, W., Zhou, K. -B., & Dai, H. -F. (2019). Three New 2-(2-Phenylethyl)chromone Derivatives of Agarwood Originated from Gyrinops salicifolia. Molecules, 24(3), 576. https://doi.org/10.3390/molecules24030576