Temperature-Dependent Evolution of Raman Spectra of Methylammonium Lead Halide Perovskites, CH3NH3PbX3 (X = I, Br)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Temperature-Dependent Evolution of the Raman Spectrum of MAPbBr3
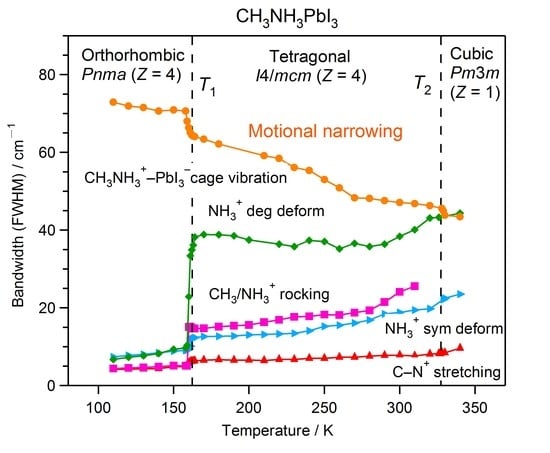
2.2. Temperature Evolution of the Raman Spectrum of MAPbI3
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snaith, H.J. Present status and future prospects of perovskite photovoltaics. Nat. Mater. 2018, 17, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic‒organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.I.; Hertz, L.M. High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Poglitsch, A.; Weber, D. Dynamic disorder in methylammoniumtrihalogenoplumbates (II) observed by millimeter-wave spectroscopy. J. Chem. Phys. 1987, 87, 6373–6378. [Google Scholar] [CrossRef]
- Swainson, I.P.; Hammond, R.P.; Soullière, C.; Knop, O.; Massa, W. Phase transitions in the perovskite methylammonium lead bromide, CH3ND3PbBr3. J. Solid State Chem. 2003, 176, 97–104. [Google Scholar] [CrossRef]
- Mashiyama, H.; Kawamura, Y.; Kubota, Y. The anti-polar structure of CH3NH3PbBr3. J. Korean Phys. Soc. 2007, 51, 850–853. [Google Scholar] [CrossRef]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitized solar cell applications. J. Mater. Chem. A 2013, 1, 5628–5641. [Google Scholar] [CrossRef]
- Onoda-Yamamuro, N.; Matsuo, T.; Suga, H. Calorimetric and IR spectroscopic studies of phase transitions in methylammonium trihalogenoplumbates (II). J. Phys. Chem. Solids 1990, 51, 1383–1395. [Google Scholar] [CrossRef]
- Yamada, K.; Hino, S.; Hirose, S.; Yamane, Y.; Turkevych, I.; Urano, T.; Tomiyasu, H.; Yamagishi, H.; Aramaki, S. Static and dynamic structures of perovskite halides ABX3 (B = Pb, Sn) and their characteristic semiconducting properties by a Hückel analytical calculation. Bull. Chem. Soc. Jpn. 2018, 91, 1196–1204. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Frost, J.M.; McMahon, A.P.; Sakai, V.G.; Kockelmann, W.; Law, C.; Li, X.; Foglia, F.; Walsh, A.; O’Regan, B.C.; et al. The dynamics of methylammonium ions in hybrid organic–inorganic perovskite solar cells. Nat. Commun. 2015, 6, 7124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Foley, B.J.; Ipek, B.; Tyagi, M.; Copley, J.R.D.; Brown, C.M.; Choi, J.J.; Lee, S.-H. Rotational dynamics of organic cations in the CH3NH3PbI3 perovskite. Phys. Chem. Chem. Phys. 2015, 17, 31278–31286. [Google Scholar]
- Mosconi, E.; Quarti, C.; Ivanovska, T.; Ruani, G.; De Angelis, F. Structural and electronic properties of organo-halide lead perovskites: a combined IE-spectroscopy and ab initio molecular dynamics investigation. Phys. Chem. Chem. Phys. 2014, 16, 16137–16144. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.T.; Weber, O.J.; Henry, P.F.; Di Pumpo, A.M.; Hansen, T.C. Complete structure and cation orientation in the perovskite photovoltaic methylammonium lead iodide between 100 and 352 K. Chem. Commun. 2015, 51, 4180–4183. [Google Scholar]
- Umebayashi, T.; Asai, K.; Kondo, T.; Nakao, A. Electronic structures of lead iodide based low-dimensional crystals. Phys. Rev. B 2003, 67, 155405. [Google Scholar] [CrossRef]
- Mosconi, E.; Amat, A.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. First-principles modeling of mixed halide organometal perovskites for photovoltaic applications. J. Phys. Chem. C 2013, 117, 13902–13913. [Google Scholar] [CrossRef]
- Filippetti, A.; Mattoni, A. Hybrid perovskites for photovoltaics: insights from first principles. Phys. Rev. B 2014, 89, 125203. [Google Scholar] [CrossRef]
- Grancini, G.; Marras, S.; Prato, M.; Giannini, C.; Quarti, C.; De Angelis, F.; De Bastiani, M.; Eperon, G.E.; Snaith, H.J.; Manna, L.; et al. The impact of the crystallization processes on the structural and optical properties of hybrid perovskite films for photovoltaics. J. Phys. Chem. Lett. 2014, 5, 3836–3842. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.R.; Eperon, G.E.; Snaith, H.J.; Giustino, F. Steric engineering of metal-halide perovskites with tunable optical band gaps. Nat. Commun. 2014, 5, 5757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Even, J.; Pedesseau, L.; Katan, C.; Kepenekian, M.; Lauret, J.-S.; Sapori, D.; Deleporte, E. Solid-state physics perspective on hybrid perovskite semiconductors. J. Phys. Chem. C 2015, 119, 10161–10177. [Google Scholar] [CrossRef]
- Park, J.-S.; Choi, S.; Yan, Y.; Yang, Y.; Luther, J.M.; Wei, S.-H.; Parilla, P.; Zhu, K. Electronic structure and optical properties of α-CH3NH3PbBr3 perovskite single crystal. J. Phys. Chem. Lett. 2015, 6, 4304–4308. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Liu, M.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Homogeneous emission line broadening in the organo lead halide perovskite CH3NH3PbI3−xClx. J. Phys. Chem. Lett. 2014, 5, 1300–1306. [Google Scholar] [CrossRef]
- Delugas, P.; Filippetti, A.; Mattoni, A. Methylammonium fragmentation in amines as source of localized trap levels and the heating role of Cl in hybrid lead-iodide perovskites. Phys. Rev. B 2015, 92, 045301. [Google Scholar] [CrossRef]
- Liu, J.; Prezhdo, O.V. Chlorine doping reduces electron–hole recombination in lead iodide perovskites: time-domain ab initio analysis. J. Phys. Chem. Lett. 2015, 6, 4463–4469. [Google Scholar] [CrossRef]
- Umari, P.; Mosconi, E.; De Angelis, F. Infrared dielectric screening determines the low exciton binding energy of metal-halide perovskites. J. Phys. Chem. Lett. 2018, 9, 620–627. [Google Scholar] [CrossRef]
- Kirchartz, T.; Markvart, T.; Rau, U.; Egger, D.A. Impact of small phonon energies on the charge-carrier lifetimes in metal-halide perovskites. J. Phys. Chem. Lett. 2018, 9, 939–946. [Google Scholar] [CrossRef]
- Glaser, T.; Müller, C.; Sendner, M.; Krekeler, C.; Semonin, O.E.; Hull, T.D.; Yaffe, O.; Owen, J.S.; Kowalsky, W.; Pucci, A.; et al. Infrared spectroscopic study of vibrational modes in methylammonium lead halide perovskites. J. Phys. Chem. Lett. 2015, 6, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Bakulin, A.A.; Selig, O.; Bakker, H.J.; Rezus, Y.L.A.; Müller, C.; Glaser, T.; Lovrincic, R.; Sun, Z.; Chen, Z.; Walsh, A.; et al. Real-time observation of organic cation reorientation in methylammonium lead iodide perovskites. J. Phys. Chem. Lett. 2015, 6, 3663–3669. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Osorio, M.A.; Milot, R.L.; Filip, M.R.; Patel, J.B.; Herz, L.M.; Johnston, M.B.; Giustino, F. Vibrational properties of the organic–inorganic halide perovskite CH3NH3PbI3 from theory and experiment: factor group analysis, first-principles calculations, and low-temperature infrared spectra. J. Phys. Chem. C 2015, 119, 25703–25718. [Google Scholar] [CrossRef]
- Capitani, F.; Marini, C.; Caramazza, S.; Dore, P.; Pisanu, A.; Malavasi, L.; Nataf, L.; Baudelet, F.; Brubach, J.-B.; Roy, P.; et al. Locking of methylammonium by pressure-enhanced H-bonding in (CH3NH3)PbBr3 hybrid perovskite. J. Phys. Chem. C 2017, 121, 28125–28131. [Google Scholar] [CrossRef]
- Schuck, G.; Többens, D.M.; Koch-Müller, M.; Efthimiopoulos, I.; Schorr, S. Infrared spectroscopic study of vibrational modes across the orthorhombic–tetragonal phase transition in methylammonium lead halide single crystals. J. Phys. Chem. C 2018, 122, 5227–5237. [Google Scholar] [CrossRef]
- Quarti, C.; Grancini, G.; Mosconi, E.; Bruno, P.; Ball, J.M.; Lee, M.M.; Snaith, H.J.; Petrozza, A.; De Angelis, F. The Raman spectrum of the CH3NH3PbI3 hybrid perovskite: interplay of theory and experiment. J. Phys. Chem. Lett. 2014, 5, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ledinský, M.; Löper, P.; Niesen, B.; Holovský, J.; Moon, S.J.; Yum, J.H.; De Wolf, S.; Fejfar, A.; Ballif, C. Raman spectroscopy of organic-inorganic halide perovskites. J. Phys. Chem. Lett. 2015, 6, 401–406. [Google Scholar] [CrossRef]
- Brivio, F.; Frost, J.M.; Skelton, J.M.; Jackson, A.J.; Weber, O.J.; Weller, M.T.; Goñi, A.R.; Leguy, A.M.A.; Barnes, P.R.F.; Walsh, A. Lattice dynamics and vibrational spectra of the orthorhombic, tetragonal, and cubic phases of methylammonium lead iodide. Phys. Rev. B 2015, 92, 144308. [Google Scholar] [CrossRef]
- Niemann, R.G.; Kontos, A.G.; Palles, D.; Kamitsos, E.I.; Kaltzoglou, A.; Brivio, F.; Falaras, P.; Cameron, P.J. Halogen effects on ordering and bonding of CH3NH3+ in CH3NH3PbX3 (X = Cl, Br, I) hybrid perovskites: Vibrational spectroscopic study. J. Phys. Chem. C 2016, 120, 2509–2519. [Google Scholar] [CrossRef]
- Ivanovska, T.; Quarti, C.; Grancini, G.; Petrozza, A.; De Angelis, F.; Milani, A.; Ruani, G. Vibrational response of methylammonium lead iodide: from cation dynamics to phonon–phonon interactions. ChemSusChem 2016, 9, 2994–3004. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Goñi, A.R.; Frost, J.M.; Skelton, J.; Brivio, F.; Rodríguez-Martínez, X.; Weber, O.J.; Pallipurath, A.; Alonso, M.I.; Campoy-Quiles, M.; et al. Dynamic disorder, phonon lifetimes, and the assignments of modes to the vibrational spectra of methylammonium lead halide perovskites. Phys. Chem. Chem. Phys. 2016, 18, 27051–27066. [Google Scholar] [PubMed]
- Létoublon, A.; Paofai, S.; Rufflé, B.; Bourges, P.; Hehlen, B.; Michel, T.; Ecolivet, C.; Durand, O.; Cordier, S.; Katan, C.; et al. Elastic constants, optical phonons, and molecular relaxations in the high temperature plastic phase of the CH3NH3PbBr3 hybrid perovskite. J. Phys. Chem. Lett. 2016, 7, 3776–3784. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Osorio, M.A.; Lin, Q.; Phillips, R.T.; Milot, R.L.; Herz, L.M.; Johnston, M.B.; Giustino, F. Raman spectrum of the organic–inorganic halide perovskite CH3NH3PbI3 from first principles and high-resolution low-temperature Raman measurements. J. Phys. Chem. C 2018, 122, 21703–21717. [Google Scholar] [CrossRef]
- Mattoni, A.; Filippetti, A.; Saba, M.I.; Caddeo, C.; Delugas, P. Temperature evolution of methylammonium trihalide vibrations at the atomic scale. J. Phys. Chem. Lett. 2016, 7, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Oxton, I.A.; Knop, O.; Duncan, J.L. The infrared spectrum and force field of the methylammonium ion in (CH3NH3)2PtCl6. J. Mol. Struct. 1977, 38, 25–32. [Google Scholar] [CrossRef]
- Maalej, A.; Abid, Y.; Kallel, A.; Daoud, A.; Lautié, A.; Romain, F. Phase transitions and crystal dynamics in the cubic perovskite CH3NH3PbCl3. Solid State Commun. 1997, 103, 279–284. [Google Scholar] [CrossRef]
- Kasahara, M.; Kaung, P.; Yagi, T. Anomaly of the Raman linewidth of the internal ν2 mode near the phase transition point of K3D(SO4)2. J. Phys. Soc. Jpn. 1994, 63, 441–444. [Google Scholar] [CrossRef]
- Yamada, K.; Kawaguchi, H.; Matsui, T. Structural phase transition and electrical conductivity of the perovskite CH3NH3Sn1−xPbxBr3 and CsSnBr3. Bull. Chem. Soc. Jpn. 1990, 63, 2521–2525. [Google Scholar] [CrossRef]
Sample Availability: Samples of the perovskite crystals are not available from the authors. |




| CH3NH3PbBr3 | ||||||
| orthorhombic | ———— | tetragonal I | ———— | tetragonal II | ———— | cubic |
| Pnma: D2h Z = 4 | 148.8 K | P4/mmm: D4 h Z = 1 | 154.0 K | I4/mcm: D4 h Z = 4 | 236.3 K | : O h Z = 1 |
| CH3NH3PbI3 | ||||||
| orthorhombic | ————————————————— | tetragonal | ———— | cubic | ||
| Pnma: D2h Z = 4 | 161.4 K | I4/mcm: D4 h Z = 4 | 330.4 K | : O h Z = 1 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakada, K.; Matsumoto, Y.; Shimoi, Y.; Yamada, K.; Furukawa, Y. Temperature-Dependent Evolution of Raman Spectra of Methylammonium Lead Halide Perovskites, CH3NH3PbX3 (X = I, Br). Molecules 2019, 24, 626. https://doi.org/10.3390/molecules24030626
Nakada K, Matsumoto Y, Shimoi Y, Yamada K, Furukawa Y. Temperature-Dependent Evolution of Raman Spectra of Methylammonium Lead Halide Perovskites, CH3NH3PbX3 (X = I, Br). Molecules. 2019; 24(3):626. https://doi.org/10.3390/molecules24030626
Chicago/Turabian StyleNakada, Kousuke, Yuki Matsumoto, Yukihiro Shimoi, Koji Yamada, and Yukio Furukawa. 2019. "Temperature-Dependent Evolution of Raman Spectra of Methylammonium Lead Halide Perovskites, CH3NH3PbX3 (X = I, Br)" Molecules 24, no. 3: 626. https://doi.org/10.3390/molecules24030626
APA StyleNakada, K., Matsumoto, Y., Shimoi, Y., Yamada, K., & Furukawa, Y. (2019). Temperature-Dependent Evolution of Raman Spectra of Methylammonium Lead Halide Perovskites, CH3NH3PbX3 (X = I, Br). Molecules, 24(3), 626. https://doi.org/10.3390/molecules24030626