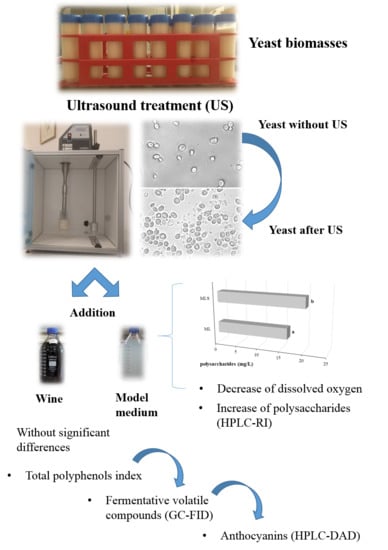
Sonication of Yeast Biomasses to Improve the Ageing on Lees Technique in Red Wines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Ultrasound Treatment on Wet Lees: Polysaccharides Released during Cellular Autolysis and Decanting Time of Hydroalcoholic Solutions
2.2. Dissolved Oxygen throughout Ageing
2.3. Volatile Acidity, Total Polyphenol Index, and Chromatic Characteristics, throughout Ageing
2.4. Evolution of Anthocyanins during Ageing
2.5. Evolution of Fermentative Volatile Compounds during Ageing
2.6. Sensory Analysis
3. Materials and Methods
3.1. Yeast Lees Biomass
3.2. Red Wine and Hydroalcoholic Solution
3.3. Ultrasound Treatment
3.4. Samples Preparation
3.5. Polysaccharides Analysis (HPLC-RI)
3.6. Volatile Acidity and Spectrophotometric Analyses
3.7. Analysis of Dissolved Oxygen
3.8. Anthocyanins and Pyranoanthocyanins
3.9. Fermentative Volatile Compounds
3.10. Sensory Analysis
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alexandre, H.; Guilloux-Benatier, M. Yeast Autolysis in Sparkling Wine—A Review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Hernández, T.; Estrella, I.; Carlavilla, D.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Phenolic Compounds in Red Wine Subjected to Industrial Malolactic Fermentation and Ageing on Lees. Anal. Chim. Acta 2006, 563, 116–125. [Google Scholar] [CrossRef]
- Moreno-Arribas, V.; Pueyo, E.; Nieto, F.; Martın-Alvarez, P.; Polo, M.C. Influence of the Polysaccharides and the Nitrogen Compounds on Foaming Properties of Sparkling Wines. Food Chem. 2000, 70, 309–317. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; Calderón, F.; Suárez-Lepe, J. New Genera of Yeasts for Over-Lees Aging of Red Wine. Food Chem. 2009, 112, 432–441. [Google Scholar] [CrossRef]
- Jiménez Moreno, N.; Ancín Azpilicueta, C. Binding of Oak Volatile Compounds by Wine Lees during Simulation of Wine Ageing. LWT - Food Sci. Technol. 2007, 40, 619–624. [Google Scholar]
- Tao, Y.; García, J.F.; Sun, D. Advances in Wine Aging Technologies for Enhancing Wine Quality and Accelerating Wine Aging Process. Crit. Rev. Food Sci. Nutr. 2014, 54, 817–835. [Google Scholar] [CrossRef]
- Babayan, T.; Bezrukov, M. Autolysis in Yeasts. Acta Biotechnol. 1985, 5, 129–136. [Google Scholar] [CrossRef]
- Kulkarni, P.; Loira, I.; Morata, A.; Tesfaye, W.; González, M.C.; Suárez-Lepe, J.A. Use of Non-Saccharomyces Yeast Strains Coupled with Ultrasound Treatment as a Novel Technique to Accelerate Ageing on Lees of Red Wines and its Repercussion in Sensorial Parameters. LWT - Food Sci. Technol. 2015, 64, 1255–1262. [Google Scholar] [CrossRef]
- Awad, T.; Moharram, H.; Shaltout, O.; Asker, D.; Youssef, M. Applications of Ultrasound in Analysis, Processing and Quality Control of Food: A Review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- García Martín, J.F.; Sun, D. Ultrasound and Electric Fields as Novel Techniques for Assisting the Wine Ageing Process: The State-of-the-Art Research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Hernanz Vila, D.; Heredia Mira, F.J.; Beltran Lucena, R.; Fernández Recamales, M. Optimization of an Extraction Method of Aroma Compounds in White Wine using Ultrasound. Talanta 1999, 50, 413–421. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; González, C.; Callejo, M.J.; Suárez-Lepe, J.A. Emerging Preservation Technologies in Grapes for Winemaking. Trends Food Sci. Technol. 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.; Sun, D. Ultrasound-Assisted Extraction of Phenolics from Wine Lees: Modeling, Optimization and Stability of Extracts during Storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C. The Effects of Different Accelerating Techniques on Maize Wine Maturation. Food Chem. 2004, 86, 61–68. [Google Scholar] [CrossRef]
- Masuzawa, N.; Ohdaira, E.; Ide, M. Effects of Ultrasonic Irradiation on Phenolic Compounds in Wine. Jpn. J. Appl. Phys. 2000, 39, 2978. [Google Scholar] [CrossRef]
- García Martín, J.F.; Guillemet, L.; Feng, C.; Sun, D. Cell Viability and Proteins Release during Ultrasound-Assisted Yeast Lysis of Light Lees in Model Wine. Food Chem. 2013, 141, 934–939. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Loira, I.; Morata, A.; González, C.; Suárez-Lepe, J.A.; Cuerda, R. Application of Ultrasound to Improve Lees Ageing Processes in Red Wines. Food Chem. 2018, 261, 157–163. [Google Scholar] [CrossRef]
- Tudela, R.; Gallardo-Chacón, J.J.; Rius, N.; López-Tamames, E.; Buxaderas, S. Ultrastructural Changes of Sparkling Wine Lees during Long-Term Aging in Real Enological Conditions. FEMS Yeast Res. 2012, 12, 466–476. [Google Scholar] [CrossRef]
- Giovani, G.; Rosi, I. Release of Cell Wall Polysaccharides from Saccharomyces Cerevisiae Thermosensitive Autolytic Mutants during Alcoholic Fermentation. Int. J. Food Microbiol. 2007, 116, 19–24. [Google Scholar] [CrossRef]
- Brenna, O.V.; Pagliarini, E. Multivariate Analysis of Antioxidant Power and Polyphenolic Composition in Red Wines. J. Agric. Food Chem. 2001, 49, 4841–4844. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Chacón, J.J.; Vichi, S.; Urpí, P.; López-Tamames, E.; Buxaderas, S. Antioxidant Activity of Lees Cell Surface during Sparkling Wine Sur Lie Aging. Int. J. Food Microbiol. 2010, 143, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Mazauric, J.; Salmon, J. Interactions between Yeast Lees and Wine Polyphenols during Simulation of Wine Aging: I. Analysis of Remnant Polyphenolic Compounds in the Resulting Wines. J. Agric. Food Chem. 2005, 53, 5647–5653. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-da-Silva, J.; Laureano, O.; González, M.; Suárez-Lepe, J. Effect of Saccharomyces Strains on the Quality of Red Wines Aged on Lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; González, C.; Suárez-Lepe, J.A. Formation of Vinylphenolic Pyranoanthocyanins by Selected Yeasts Fermenting Red Grape Musts Supplemented with Hydroxycinnamic Acids. Int. J. Food Microbiol. 2007, 116, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.; Versini, G. Influence of nitrogen compounds in grapes on aroma compounds of wines. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 1659–1694. [Google Scholar]
- Ribéreau-Gayon, J.; Peynaud, E.; Sudraud, P.; Ribéreau-Gayon, P. Tratado De Enologia: Ciencias Y Tecnicas Del Vino: Analisis Y Control De Los Vinos; Hemisferio Sur: Buenos Aires, Argentina, 1980. [Google Scholar]
- Glories, Y. La Couleur Des Vins Rouges: 2e. Partie: Mesure, Origine Et Interpretation. Connaissance de la Vigne et du Vin 1984, 18. [Google Scholar] [CrossRef]
- Abalos, D.; Vejarano, R.; Morata, A.; González, C.; Suárez-Lepe, J.A. The use of Furfural as a Metabolic Inhibitor for Reducing the Alcohol Content of Model Wines. Eur. Food Res. Technol. 2011, 232, 663–669. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Fresno, J.M.; Morata, A.; Escott, C.; Loira, I.; Cuerda, R.; Suárez-Lepe, J.A. Sonication of Yeast Biomasses to Improve the Ageing on Lees Technique in Red Wines. Molecules 2019, 24, 635. https://doi.org/10.3390/molecules24030635
del Fresno JM, Morata A, Escott C, Loira I, Cuerda R, Suárez-Lepe JA. Sonication of Yeast Biomasses to Improve the Ageing on Lees Technique in Red Wines. Molecules. 2019; 24(3):635. https://doi.org/10.3390/molecules24030635
Chicago/Turabian Styledel Fresno, Juan Manuel, Antonio Morata, Carlos Escott, Iris Loira, Rafael Cuerda, and José Antonio Suárez-Lepe. 2019. "Sonication of Yeast Biomasses to Improve the Ageing on Lees Technique in Red Wines" Molecules 24, no. 3: 635. https://doi.org/10.3390/molecules24030635
APA Styledel Fresno, J. M., Morata, A., Escott, C., Loira, I., Cuerda, R., & Suárez-Lepe, J. A. (2019). Sonication of Yeast Biomasses to Improve the Ageing on Lees Technique in Red Wines. Molecules, 24(3), 635. https://doi.org/10.3390/molecules24030635