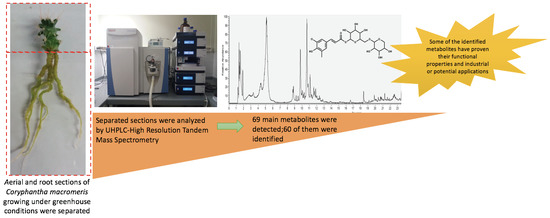
Phytochemical Profiling of Coryphantha macromeris (Cactaceae) Growing in Greenhouse Conditions Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Sample Preparation
3.3. UHPLC-PDA-HESI-Orbitrap-MS/MS Conditions
MS Parameters
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santos-Díaz, M.S.; Pérez-Molphe, E.; Ramírez-Malagón, R.; Núñez-Palenius, H.G.; Ochoa- Alejo, N. Mexican Threatened Cacti: Current Status and Strategies for Their Conservation. In Species Diversity and Extinction; Tepper, G.H., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 1–60. [Google Scholar]
- Mauseth, J.D. Biogeography and biodiversity of cacti. Cactus Succul. J. 2016, 88, 46. [Google Scholar] [CrossRef]
- Martins, C.; Oliveira, R.; Mendonça Filho, C.V.; Teixeira Lopes, L.; Assunção Silveira, R.; Pereira de Silva, J.A.; Aguiar, L.M.S.; Antonini, Y. Reproductive biology of Cipocereus minensis (Cactaceae)—A columnar cactus endemic to rupestrian fields of a Neotropical savannah. Flora 2016, 218, 62–67. [Google Scholar] [CrossRef]
- Saenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia spp. mucilage’s: A functional component with industrial perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Lillo, L.E.; Sáenz, C.; Urzúa, C.C.; Zárate, O. Chemical characterization of the mucilage from fruits of Opuntia ficus indica. Carbohydr. Polym. 2006, 63, 263–267. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Valente, L.M.M.; da Paixão, D.; do Nascimento, A.C.; dos Santos, P.F.P.; Scheinvar, L.A.; Moura, M.R.L.; Tinoco, L.W.; Gomes, L.N.F.; da Silva, J.F.M. Antiradical activity, nutritional potential and flavonoids of the cladodes of Opuntia monacantha (Cactaceae). Food Chem. 2010, 123, 1127–1131. [Google Scholar] [CrossRef]
- Zampini, I.C.; Ordoñez, R.; Giannini, N.P.; Blendinger, P.G.; Isla, M.I. Nutraceutical properties and toxicity studies of fruits from four Cactaceae species grown in Argentine Northwestern. Food Res. Int. 2011, 44, 2345–2351. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Khemakhem, B.; Yangui, T.; Attia, H. Variation in chemical composition and biological activities of two species of Opuntia flowers at four stages of flowering. Ind. Crops Prod. 2012, 37, 34–40. [Google Scholar] [CrossRef]
- Starha, R.; Chybidziurová, A.; Lance, Z. Alkaloids of the genus Turbinicarpus (Cactaceae). Biochem. Syst. Ecol. 1999, 27, 839–841. [Google Scholar] [CrossRef]
- Ogunbodede, O.; McCombs, D.; Trout, K.; Daley, P.; Terry, M. New mescaline concentrations from 14 taxa/cultivars of Echinopsis spp. (Cactaceae) (“San Pedro”) and their relevance to shamanic practice. J. Ethnopharmacol. 2010, 131, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.d.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; Barba de la Rosa, A.P. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.M.; Bronze, M.R. Contribution to the characterization of Opuntia spp. juices by LC–DAD–ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Ammar, I.; Ennouri, M.; Attia, H. Phenolic content and antioxidant activity of cactus (Opuntia ficus-indica L.) flowers are modified according to the extraction method. Ind. Crops Prod. 2015, 64, 97–104. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Saïdani Tounsi, M.; Ouerghemmi, I.; Ksouri, R.; Aidi Wannes, W.; Hammrouni, I.; Marzouk, B. HPLC-determination of phenolic composition and antioxidant capacity of cactus prickly pears seeds. Asian J. Chem. 2011, 23, 1006–1010. [Google Scholar]
- Herrera-Hernández, M.G.; Guevara-Lara, F.; Reynoso-Camacho, R.; Guzmán-Maldonado, S.H. Effects of maturity stage and storage on cactus berry (Myrtillocactus geometrizans) phenolics, vitamin C, betalains and their antioxidant properties. Food Chem. 2011, 129, 1744–1750. [Google Scholar] [CrossRef]
- Cha, M.-N.; Jun, H.-I.; Lee, W.-J.; Kim, M.-J.; Kim, M.-K.; Kim, Y.-S. Chemical composition and antioxidant activity of Korean cactus (Opuntia humifusa) fruit. Food Sci. Biotechnol. 2013, 22, 523–529. [Google Scholar] [CrossRef]
- Jun, H.-I.; Cha, M.-N.; Yang, E.-I.; Choi, D.G.; Kim, Y.-S. Physicochemical properties and antioxidant activity of Korean cactus (Opuntia humifusa) cladodes. Hortic. Environ. Biotechnol. 2013, 54, 288–295. [Google Scholar] [CrossRef]
- Werner, E.; Heilier, J.-F.; Ducruix, C.; Ezan, E.; Junot, C.; Tabet, J.-C. Mass spectrometry for the identification of the discriminating signals from metabolomics: Current status and future trends. J. Chromatogr. B 2008, 871, 143–163. [Google Scholar] [CrossRef]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrAC Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Pérez-Míguez, R.; Montero, L.; Herrero, M. Application of mass spectrometry-based metabolomics approaches for food safety, quality and traceability. TrAC Trends Anal. Chem. 2017, 93, 102–118. [Google Scholar] [CrossRef]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Ram, S. Chemotaxonomy: A tool for plant classification. J. Med. Plants Stud. 2016, 4, 90–93. [Google Scholar]
- Dicht, R.; Lüthy, A. Coryphantha—Cacti of Mexico and Southern USA; Springer: Berlin/Heidelberg, Germany, 2005; pp. 3–5. [Google Scholar] [CrossRef]
- Pérez-Molphe-Balch, E.; Pérez-Reyes, M.E.; Dávila-Figueroa, C.A.; Villalobos-Amador, E. In vitro propagation of three species of columnar cacti from the Sonoran desert. HortScience 2002, 37, 693–696. [Google Scholar] [CrossRef]
- Pérez-Molphe-Balch, E.; Santos-Díaz, M.d.S.; Ramírez-Malagón, R.; Ochoa-Alejo, N. Tissue culture of ornamental cacti. Sci. Agric. 2015, 72, 540–561. [Google Scholar] [CrossRef] [Green Version]
- Batis, A.; Rojas, M. El peyote y otros cactos alucinógenos de México. CONABIO Biodiversitas 2002, 40, 12–17. [Google Scholar]
- Keller, W.J.; McLaughlin, J.L. Cactus Alkaloids XIII: Isolation of (−)—Normacromerine from Coryphantha macromeris var. runyonii. J. Pharm. Sci. 1972, 61, 147–148. [Google Scholar] [CrossRef]
- Kikuchi, H.; Uchiyama, N.; Ogata, J.; Kikura-Hanajiri, R.; Goda, Y. Chemical constituents and DNA sequence analysis of a psychotropic herbal product. Forensic Toxicol. 2010, 28, 77–83. [Google Scholar] [CrossRef]
- La Barbera, G.; Capriotti, A.L.; Cavaliere, C.; Montone, C.M.; Piovesana, S.; Samperi, R.; Zenezini Chiozzi, R.; Laganà, A. Liquid chromatography-high resolution mass spectrometry for the analysis of phytochemicals in vegetal-derived food and beverages. Food Res. Int. 2017, 100, 28–52. [Google Scholar] [CrossRef]
- Cornejo, A.; Salgado, F.; Caballero, J.; Vargas, R.; Simirgiotis, M.; Areche, C. Secondary metabolites in Ramalina terebrata detected by UHPLC/ESI/MS/MS and identification of parietin as Tau protein inhibitor. Int. J. Mol. Sci. 2016, 17, 1303. [Google Scholar] [CrossRef]
- Simirgiotis, M.; Quispe, C.; Borquez, J.; Areche, C.; Sepulveda, B. Fast detection of phenolic compounds in extracts of easter pears (Pyrus communis) from the Atacama desert by Ultra High-Performance Liquid Chromatography and Mass Spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef]
- Simirgiotis, M.; Quispe, C.; Areche, C.; Sepulveda, B. Phenolic compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) analyzed by UHPLC-Q/Orbitrap/MS/MS and its antioxidant properties. Molecules 2016, 21, 245. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Masuoka, C.; Koto, M.; Tateishi, M.; Komatsu, H.; Kobayashi, H.; Igoshi, K.; Ito, Y.; Okawa, M.; Nohara, T. Antioxidant ortho-benzoyloxyphenyl acetic acid ester, vaccihein A, from the fruit of rabbiteye blueberry (Vaccinium ashei). Chem. Pharm. Bull. 2002, 50, 1416–1417. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Escobar, C.A.; Priego-Capote, F.; Luque de Castro, M.D. Characterization of lemon (Citrus limon) polar extract by liquid chromatography-tandem mass spectrometry in high resolution mode. J. Mass Spectrom. 2015, 50, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Barros, L.; Ramírez-Moreno, E.; Santos-Buelga, C.; Ferreira, I.C.F.R. Exploring xoconostle by-products as sources of bioactive compounds. Food Res. Int. 2014, 65(Part C), 437–444. [Google Scholar] [CrossRef] [Green Version]
- Vankudothu, N.; Anwar, S.Y. Molecular interaction studies of Thespesia populnea extracts and their analogs on therapeutic targets by molecular docking. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 82–85. [Google Scholar]
- Chang, B.-B.; Zhang, L.; Cao, W.-W.; Cao, Y.; Yang, W.-L.; Wang, Y.; Chen, Y.-C.; Liu, X.-Q. Pharmacokinetic interactions induced by content variation of major water-soluble components of Danshen preparation in rats. Acta Pharmacol. Sin. 2010, 31, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Allai, L.; Karym, E.M.; El Amiri, B.; Nasser, B.; Essamad, A.; Terzioğlu, P.; Ertas, A.; Öztürk, M. Evaluation of antioxidant activity and phenolic composition of Opuntia ficus-indica cladodes collected from Moroccan Settat region. Eurasian J. Anal. Chem. 2016, 12, 105–117. [Google Scholar] [CrossRef]
- Takeda, T.; Gonda, R.; Hatano, K. Constitution of Lucumin and its related glycosides from Calocarpum sapota Merrill. Chem. Pharm. Bull. 1997, 45, 697–699. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Quifer-Rada, P.; Andrés, A.; Lamuela-Raventós, R. Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). J. Funct. Food. 2016, 23, 370–377. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, K.-J.; Koh, D.-J.; Kim, S.-H.; Park, S.J.; Ryu, J.H.; Kim, D.-G.; Lee, J.-Y.; Lee, K.-T. Anti-Inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-κappaB inactivation. J. Agric. Food. Chem. 2008, 56, 10265–10272. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Piasecka, A.; Gryszczyńska, A.; Sawikowska, A.; Pietrowiak, A.; Opala, B.; Mikołajczak, P.Ł.; Kujawski, R.; Kachlicki, P.; Buchwald, W.; et al. Determination of phenolic compounds and diterpenes in roots of Salvia miltiorrhiza and Salvia przewalskii by two LC–MS tools: Multi-stage and high resolution tandem mass spectrometry with assessment of antioxidant capacity. Phytochem. Lett. 2017, 20, 331–338. [Google Scholar] [CrossRef]
- Maier, C.; Conrad, J.; Carle, R.; Weiss, J.; Schweiggert, R.M. Phenolic constituents in commercial aqueous Quillaja (Quillaja saponaria Molina) wood extracts. J. Agric. Food. Chem. 2015, 63, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Qin, K.; Ding, F.; Huang, Y.; Wang, X.; Cai, B. Identification and differentiation of major components in three different “Sheng-ma” crude drug species by UPLC/Q-TOF-MS. Acta Pharm. Sin. B 2017, 7, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, C.T.; Nasiya, K.K.; Balachandran, I. Isolation and mass spectroscopic characterization of phytochemicals from the bark of Acacia leucophloea (Roxb.) Willd. Spectrosc. Lett. 2016, 49, 391–395. [Google Scholar] [CrossRef]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic composition, antioxidant and anti-inflammatory activities of extracts from Moroccan Opuntia ficus-indica flowers obtained by different extraction methods. Ind. Crops Prod. 2014, 62, 412–420. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Quispe, C.; Camarantin Soriano, M.d.P.; Fuentes Gonzalez, J.; Hüneke, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and characterization of constituents in copao fruits (Eulychnia acida Phil., Cactaceae) by HPLC–DAD–MS/MSn. Food Res. Int. 2014, 62, 286–298. [Google Scholar] [CrossRef]
- Serra, A.T.; Poejo, J.; Matias, A.A.; Bronze, M.R.; Duarte, C.M.M. Evaluation of Opuntia spp. derived products as antiproliferative agents in human colon cancer cell line (HT29). Food Res. Int. 2013, 54, 892–901. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Shimada, H.; Yoshizumi, S.; Saka, M.; Yamahara, J.; Matsuda, H. Medicinal foodstuffs. XIV. On the bioactive constituents of Moroheiya. (2): New fatty Acids, Corchorifatty Acids A, B, C, D, E, and F, from the leaves of Corchorus olitorius L. (Tiliaceae): Structures and inhibitory effect on NO production in mouse peritoneal macrophages. Chem. Pharm. Bull. 1998, 46, 1008–1014. [Google Scholar] [PubMed]
- Kim, C.S.; Kwon, O.W.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Five new oxylipins from Chaenomeles sinensis. Lipids 2014, 49, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yamaguchi, Y.; Abe, N.; Uyehara, T.; Namai, T.; Kodama, M.; Shiobara, Y. Structure and synthesis of unsaturaded trihydroxy C18 fatty: Acids in rice plants suffering from rice blast disease. Tetrahedron Lett. 1985, 26, 2357–2360. [Google Scholar] [CrossRef]
- Liu, M.; Li, P.; Zeng, X.; Wu, H.; Su, W.; He, J. Identification and pharmacokinetics of multiple potential bioactive constituents after oral administration of Radix Astragali on cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Mol. Sci. 2015, 16, 5047–5071. [Google Scholar] [CrossRef] [PubMed]
- Burchacka, E.; Potaczek, P.; Paduszyński, P.; Karłowicz-Bodalska, K.; Han, T.; Han, S. New effective azelaic acid liposomal gel formulation of enhanced pharmaceutical bioavailability. Biomed. Pharmacother. 2016, 83, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Mayer-da-Silva, A.; Gollnick, H.; Detmar, M.; Gassmuller, J.; Parry, A.; Muller, R.; Orfanos, C.E. Effects of azelaic acid on sebaceous gland, sebum excretion rate and keratinization pattern in human skin. An in vivo and in vitro study. Acta Dermato Venereol. Suppl. 1989, 143, 20–30. [Google Scholar]
- Lowe, N.J.; Rizk, D.; Grimes, P.; Billips, M.; Pincus, S. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin. Ther. 1998, 20, 945–959. [Google Scholar] [CrossRef]
- Yang, X.; Wong, M.; Wang, N.; Chan, A.S.; Yao, X. A new eudesmane derivative and a new fatty acid ester from Sambucus williamsii. Chem. Pharm. Bull. 2006, 54, 676–678. [Google Scholar] [CrossRef]
- Youssef, D.T. Hyrtioerectines A-C, cytotoxic alkaloids from the red sea sponge Hyrtios erectus. J. Nat. Prod. 2005, 68, 1416–1419. [Google Scholar] [CrossRef]
- Li, H.; Yu, Y.; Wang, Z.; Geng, J.; Dai, Y.; Xiao, W.; Yao, X. Chemical profiling of Re-Du-Ning injection by ultra-performance liquid chromatography coupled with electrospray ionization tandem quadrupole time-of-flight mass spectrometry through the screening of diagnostic ions in MS(E) mode. PLoS ONE 2015, 10, e0121031. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhai, H.Y.; Tang, S.A.; Duan, H.Q. Studies on the chemical constituents of Pachysandra terminalis and their antioxidant activity. Zhong Yao Cai 2010, 33, 729–732. [Google Scholar] [PubMed]
- Kim, K.H.; Lee, K.H.; Choi, S.U.; Kim, Y.H.; Lee, K.R. Terpene and phenolic constituents of Lactuca indica L. Arch. Pharm. Res. 2008, 31, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Harada, G.; Kondo, N. Induction of phytoalexins in adzuki bean after inoculation with Phytophthora vignae f. sp. adzukicola. J. Gen. Plant Pathol. 2009, 75, 432–436. [Google Scholar] [CrossRef]
- Samoszuk, M.; Tan, J.; Chorn, G. The chalcone butein from Rhus verniciflua Stokes inhibits clonogenic growth of human breast cancer cells co-cultured with fibroblasts. BMC Complement. Altern. Med. 2005, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.-J.; Kuo, S.-C.; Chan, S.-C.; Ko, F.-N.; Teng, C.-M. Antioxidant properties of butein isolated from Dalbergia odorifera. Biochim. Biophys. Acta-Lipids Lipid Metab. 1998, 1392, 291–299. [Google Scholar] [CrossRef]
- Jayasooriya, R.G.P.T.; Molagoda, I.M.N.; Park, C.; Jeong, J.-W.; Choi, Y.H.; Moon, D.-O.; Kim, M.-O.; Kim, G.-Y. Molecular chemotherapeutic potential of butein: A concise review. Food Chem. Toxicol. 2018, 112, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiologia Plantarum 1962, 15, 473–497. [Google Scholar] [CrossRef]
Sample Availability: Samples of plant material and extracs are available from the authors. |



| Peak | Retention Time (min) | Tentative Identification | Elemental Composition [M-H]− | Theoretical Mass (m/z) | Measured Mass (m/z) | Accuracy (ppm) | MSn Ions | Plant Part | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.37 | 220, 272 | Vaccihein A | C18H17O9− | 377.08781 | 377.08600 | 4.80 | 361.09076 ([M-H-OH]−) | Root/shoot |
| 347.07724 ([M-H-OCH3]−) | |||||||||
| 319.07990 ([M-H-C2H3O2]−) | |||||||||
| 289.06958 ([M-H-C2H3O2-OCH3]−) | |||||||||
| 125.02375 ([M-H-C8H9O4-C3H5O2]−) | |||||||||
| 2 | 1.45 | 220, 272 | Dihydroxy methoxy butanoic acid | C5H9O5− | 149.04555 | 149.04498 | 3.82 | 135.02925 ([M-H-CH3]−) | Root/shoot |
| 131.03433 ([M-H-OH]−) | |||||||||
| 119.03425 ([M-H-CH3-OH]−) | |||||||||
| 103.03932 ([M-H-OCH3-OH]−) | |||||||||
| 3 | 1.52 | 220, 274 | 2-Hydroxy-succinic acid (malic acid) | C4H5O5− | 133.01390 | 133.01363 | 2.03 | 115.00297 ([M-H-OH]−) | Root/shoot |
| 4 | 1.55 | 224, 277 | Iso citric acid | C6H7O7− | 191.01973 | 191.01938 | 1.83 | 111.00790 ([M-H-CO2-2OH]−) | Root/shoot |
| 5 | 1.87 | 277 | Iso citric acid isomer | C6H7O7− | 191.01973 | 191.01938 | 1.83 | 111.00790 ([M-H-CO2-2OH]−) | Root/shoot |
| 6 | 2.35 | 220, 277 | 3,5-Dihydroxy-4-methyloxolan-2-yl methoxy-6-hydroxymethyl oxane-3,4,5-triol | C12H21O9− | 309.11911 | 309.11935 | 0.78 | 293.12424 ([M-H-OH]−) | Root |
| 279.10870 ([M-H-OH-CH3]−) | |||||||||
| 147.06578 ([M-H-C6H11O5]−) | |||||||||
| 131.07106 ([M-H-C6H11O5-OH]−) | |||||||||
| 7 | 2.74 | 224, 277 | Piscidic acid isomer | C11H11O7− | 255.05103 | 255.05098 | 0.20 | 193.05013 ([M-H-CHO2-OH]−) | Shoot |
| 165.05521 ([M-H-C2H2O3-OH]−) | |||||||||
| 135.04442 ([M-H-C2H2O3-CHO2]−) | |||||||||
| 119.04952 ([M-H-C2H2O3-CH2-OH]−) | |||||||||
| 107.04927 ([M-H-C4H4O3]−) | |||||||||
| 8 | 3.34 | 228, 277 | Piscidic acid isomer | C11H11O7− | 255.05103 | 255.05098 | 0.20 | 193.05013 ([M-H-CHO2-OH]−) | Shoot |
| 165.05521 ([M-H-C2H2O3-OH]−) | |||||||||
| 135.04442 ([M-H-C2H2O3-CHO2]−) | |||||||||
| 119.04952 ([M-H-C2H2O3-CH2-OH]−) | |||||||||
| 107.04927 ([M-H-C4H4O3]−) | |||||||||
| 9 | 4.22 | 197, 223, 278 | Protocatechuic acid hesoxide | C13H15O9− | 315.07230 | 315.07239 | 0.29 | 255.05104 ([M-H-C2H5O2]−) | Root |
| 211.06094 ([M-H-C2H5O2-CHO2]−) | |||||||||
| 153.01878 ([M-H-C6H11O5]−) | |||||||||
| 137.02385 ([M-H-C6H11O5-OH]−) | |||||||||
| 121.02895 ([M-H-C6H11O5-2OH]−) | |||||||||
| 109.02863 ([M-H-C6H11O5-CHO2]−) | |||||||||
| 10 | 4.34 | 223, 276 | Piscidic acid isomer | C11H11O7− | 255.05103 | 255.05098 | 0.20 | 193.05013 ([M-H-CHO2-OH]−) | Shoot |
| 165.05521([M-H-C2H2O3-OH]−) | |||||||||
| 135.04442 ([M-H-C2H2O3-CHO2]−) | |||||||||
| 119.04952 ([M-H-C2H2O3-CH2-OH]−) | |||||||||
| 107.04927 ([M-H-C4H4O3]−) | |||||||||
| 11 | 5.13 | 222, 275 | Piscidic acid isomer | C11H11O7− | 255.05103 | 255.05095 | 0.31 | 193.05013 ([M-H-CHO2-OH]−) | Root/shoot |
| 165.05521 ([M-H-C2H2O3-OH]−) | |||||||||
| 135.04442 ([M-H-C2H2O3-CHO2]−) | |||||||||
| 119.04952 ([M-H-C2H2O3-CH2-OH]−) | |||||||||
| 107.04927 ([M-H-C4H4O3]−) | |||||||||
| 12 | 6.02 | 222, 275 | Piscidic acid isomer | C11H11O7− | 255.05103 | 255.05101 | 0.08 | 193.05013 ([M-H-CHO2-OH]−) | Shoot |
| 165.05521([M-H-C2H2O3-OH]−) | |||||||||
| 135.04442 ([M-H-C2H2O3-CHO2]−) | |||||||||
| 119.04952 ([M-H-C2H2O3-CH2-OH]−) | |||||||||
| 107.04927 ([M-H-C4H4O3]−) | |||||||||
| 13 | 8.74 | 222, 277 | Lucuminic acid | C19H25O12− | 445.13515 | 445.13531 | 0.36 | 163.03947 ([M-H-C10H19O8-OH]−) | Shoot |
| 119.04939 ([M-H-C11H19O10-OH]−) | |||||||||
| 107.04942 ([M-H-C11H19O9-CHO2]−) | |||||||||
| 14 | 8.85 | 223, 276 | Hyrtioerectine C | C11H12NO4− | 222.07718 | 222.07703 | 0.68 | 206.08206 ([M-H-OH]−) | Root/shoot |
| 198.07718 ([M-H-C2H3]−) | |||||||||
| 180.06580 ([M-H-C2H3-OH]−) | |||||||||
| 178.08685 ([M-H-CHO2]−) | |||||||||
| 15 | 8.93 | 227, 283 | Piscidic acid isomer | C11H11O7− | 255.05103 | 255.05104 | 0.04 | 193.05013 ([M-H-CHO2-OH]−) | Shoot |
| 165.05521([M-H-C2H2O3-OH]−) | |||||||||
| 135.04442 ([M-H-C2H2O3-CHO2]−) | |||||||||
| 119.04952 ([M-H-C2H2O3-CH2-OH]−) | |||||||||
| 107.04927 ([M-H-C4H4O3]−) | |||||||||
| 16 | 9.12 | 255, 207 | Protocatechuic aldehyde | C7H5O3− | 137.02442 | 137.02386 | 4.09 | 121.02882 ([M-H-OH]−) | Root |
| 109.02884 ([M-H-COH]−) | |||||||||
| 17 | 9.19 | 230, 286 | Piscidic acid derivative | C21H27O13− | - | 487.14600 | - | 255.05110 ([piscidic acid]−) | Root/shoot |
| 193.05078 ([piscidic acid-CHO2-OH]−) | |||||||||
| 165.05516 ([piscidic acid-C2H2O3-OH]− | |||||||||
| 135.04453 ([piscidic acid-C2H2O3-CHO2]−) 107.04935 ([piscidic acid-C4H4O3]−) | |||||||||
| 18 | 9.41 | 223, 278 | Piscidic acid derivative | C20H27O13− | - | 475.14606 | - | 255.05112 ([piscidic acid]−) | Root |
| 193.05037 ([piscidic acid-CHO2-OH]−) | |||||||||
| 165.05513([piscidic acid-C2H2O3-OH]−) | |||||||||
| 135.04453 ([piscidic acid-C2H2O3-CHO2]−) | |||||||||
| 107.04923 ([piscidic acid-C4H4O3]−) | |||||||||
| 19 | 9.45 | 231, 295 | Sinapic acid derivative | C22H29O14− | - | 517.15649 | - | 223.06104 ([sinapic acid]−) | Shoot |
| 208.03767 ([sinapic acid-CH3]−) | |||||||||
| 179.07083 ([sinapic acid- CHO2]−) | |||||||||
| 164.04738 ([sinapic acid-CHO2-OH]−) | |||||||||
| 20 | 9.50 | 231, 295 | Sinapic acid | C11H11O5− | 223.06070 | 223.06102 | 1.43 | 208.03757 ([M-2H-CH3]−) | Shoot |
| 179.07094 ([M-H-CHO2]−) | |||||||||
| 164.04730 ([M-2H-CHO2-OH]−) | |||||||||
| 21 | 9.77 | 224, 276 | Syringic acid acetate | C11H11O6− | 239.05611 | 239.05594 | 0.71 | 197.04517 ([syringic acid]−) | Root/shoot |
| 195.06580 ([M-H-CHO2]−) | |||||||||
| 179.03439 ([M-H-2CH3O]−) | |||||||||
| 149.06023 [M-H-2CH3O-OH]−) | |||||||||
| 135.04456 ([M-H-CHO2-2CH3O]−) | |||||||||
| 107.04944 ([M-H-CHO2-CH3O-C2H3O2]−) | |||||||||
| 22 | 9.97 | 231, 286 | Piscidic acid derivative | C21H27O13− | - | 487.14603 | - | 255.05101 ([piscidic acid]−) | Shoot |
| 193.05025 ([piscidic acid-CHO2-OH]−) | |||||||||
| 165.05528 ([piscidic acid-C2H2O3-OH]−) | |||||||||
| 135.04456 ([piscidic acid-C2H2O3-CHO2]−) | |||||||||
| 107.04945 ([piscidic acid-C4H4O3]−) | |||||||||
| 23 | 10.04 | 233, 283 | Syringic acid acetate derivative | C19H31O8− | - | 387.20276 | - | 239.05594 ([syringic acid acetate]−) | Shoot |
| 197.04517 ([syringic acid]−) | |||||||||
| 195.06580 ([syringic acid acetate-CHO2]−) | |||||||||
| 179.03439 ([syringic acid acetate-2CH3O]−) | |||||||||
| 149.06023 ([syringic acid acetate-2CH3O-OH]−) | |||||||||
| 135.04456 ([syringic acid acetate-CHO2-2CH3O]−) | |||||||||
| 107.04944 ([syringic acid acetate-CHO2-CH3O-C2H3O2]−) | |||||||||
| 24 | 10.08 | 231, 283 | Caffeic acid | C9H7O4− | 179.03498 | 179.03477 | 1.17 | 163.03950 ([M-H-OH]−) | Root |
| 135.04510 ([M-H-CHO2]−) | |||||||||
| 109.02870 ([M-H-C3H3O2]−) | |||||||||
| 25 | 10.17 | 231, 286 | Sinapic acid hexoside | C17H21O10− | 385.11402 | 385.11438 | 0.93 | 223.06099 ([M-H-C6H11O5]−) | Shoot |
| 208.03757 ([sinapic acid-CH3]−) | |||||||||
| 179.07095 ([sinapic acid-CHO2]−) | |||||||||
| 164.04745 ([sinapic acid-CHO2-OH]−) | |||||||||
| 26 | 10.27 | 234, 283 | Propanedioic acid, [5-[[2-[(6-deoxy- α-l-galactopyranosyl) oxy] cyclohexyl] oxy]-Pentyl] | C20H33O10− | 433.20792 | 433.20825 | 0.76 | 417.21347 ([M-H-OH]−) | Shoot |
| 387.20309 ([M-H-CHO2]−) | |||||||||
| 287.15030 ([M-H-C6H11O4]−) | |||||||||
| 245.13950 ([M-H-C8H13O5]−) | |||||||||
| 131.07069 ([M-H-C14H23O6]−) | |||||||||
| 27 | 10.31 | 237, 283 | Cyclohexanecarboxylic acid, 3-[(6-deoxy-3-O-methyl-d-galactopyranosyl)oxy]-1,4,5-trihydroxy | C14H23O10− | 351.12967 | 351.13010 | 1.22 | 303.14487 ([M-H-3OH]−) | Root |
| 287.14999 ([M-H-4OH]−) | |||||||||
| 273.13449 ([M-H-3OH-CH3O]−) | |||||||||
| 28 | 10.63 | 235, 326 | Ferulic acid | C10H9O4− | 193.05063 | 193.05032 | 1.61 | 179.03455 ([M-H-CH3]−) | Root/shoot |
| 149.06050 ([M-H-CHO2]−) | |||||||||
| 163.03963 ([M-H-CH3-OH]−) | |||||||||
| 147.04456 ([M-H-CH3-2OH]−) | |||||||||
| 29 | 10.69 | 235, 327 | Ferulic acid derivative (fertaric acid) | C14H13O9− | 325.05651 | 325.05664 | 0.40 | 193.05032 ([ferulic acid]−) | Root/shoot |
| 179.03453 ([ferulic acid-CH3]−) | |||||||||
| 163.03954 ([ferulic acid-CH3-OH]−) | |||||||||
| 30 | 10.84 | 237, 291 | 7,8,11-Trihydroxyguai-4-en- 3-one 8-O-β-d-glucopyranoside | C21H33O9− | 429.21250 | 429.21335 | 1.98 | 267.16003 ([M-H-C6H11O5]−) | Shoot |
| 249.14989 ([M-H-C6H11O5-OH]−) | |||||||||
| 31 | 11.09 | 235, 286 | Cinnamic acid derivative | C8H14O6− | - | 206.08205 | - | 147.04449 ([cinnamic acid]−) | Root/shoot |
| 103.05447 ([cinnamic acid-CHO2]−) | |||||||||
| 32 | 11.22 | 227, 283 | 2-Propenoic acid, 2-methyl-, 4-[2-(2,4-dioxo-1,5-dioxaspiro [5.5]undec-3-yl)ethenyl]-6-(2,4-dioxo-1,5-dioxaspiro[5.5]undec-3-ylidene)-4-hexenyl ester | C30H34O10− | 554.21629 | 554.21448 | 3.27 | 193.05049 ([M-H-C20H25O6]−) | Root |
| 33 | 11.54 | 239, 289, 323 | Ferulic acid isomer | C10H9O4− | 193.05063 | 193.05048 | 0.78 | 179.03458 ([M-H-CH3]−) | Root/shoot |
| 149.06030 ([M-H-CHO2]−) | |||||||||
| 163.03954 ([M-H-CH3-OH]−) | |||||||||
| 34 | 11.61 | 238, 292, 323 | Ferulic acid isomer | C10H9O4− | 193.05063 | 193.05038 | 1.29 | 179.03441 ([M-H-CH3]−) | Shoot |
| 149.06026 ([M-H-CHO2]−) | |||||||||
| 163.03937 ([M-H-CH3-OH]−) | |||||||||
| 147.04457 ([M-H-CH3-2OH]−) | |||||||||
| 35 | 11.66 | 226, 282 | Ferulic acid derivative I | C24H24O5− | - | 392.16193 | - | 193.05038 ([ferulic acid]−) | Root |
| 149.06030 ([ferulic acid-CHO2]−) | |||||||||
| 163.03954 ([ferulic acid-CH3-OH]−) | |||||||||
| 36 | 11.71 | 236, 286 | Ferulic acid isomer | C10H9O4− | 193.05063 | 193.05038 | 1.29 | 179.03452 ([M-H-CH3]−) | Shoot |
| 149.06024 ([M-H-CHO2]−) | |||||||||
| 163.03937 ([M-H-CH3-OH]−) | |||||||||
| 147.04440 ([M-H-CH3-2OH]−) | |||||||||
| 37 | 11.79 | 235, 286, 380 | 2-Isoferulic piscidic acid-1-metyl ester | C22H21O10− | 445.11400 | 445.11438 | 0.85 | 255.05092 ([piscidic acid]−) | Shoot |
| 193.050380 ([ferulic acid]−) | |||||||||
| 165.05516 ([piscidic acid-C2H2O3-OH]−) | |||||||||
| 135.04440 ([piscidic acid-C2H2O3-CHO2]−) | |||||||||
| 107.04936 ([piscidic acid-C4H4O3]−) | |||||||||
| 38 | 11.86 | 240, 296, 381 | Ferulic acid derivative II | C20H29O10− | - | 429.17707 | - | 193.05029 ([ferulic acid]−) | Root/shoot |
| 179.03450 ([ferulic acid-CH3]−) | |||||||||
| 163.03937 ([ferulic acid-CH3-OH]−) | |||||||||
| 147.04440 ([ferulic acid-CH3-2OH]−) | |||||||||
| 39 | 12.18 | 283, 368 | Ferulic acid derivative III | C21H31O13− | - | 491.17731 | - | 193.05026 ([ferulic acid]−) | Shoot |
| 179.03456 ([ferulic acid-CH3]−) | |||||||||
| 163.03929 ([ferulic acid-CH3-OH]−) | |||||||||
| 147.04446 ([ferulic acid-CH3-2OH]−) | |||||||||
| 40 | 12.38 | 283 | Azelaic acid | C9H15O4− | 187.09758 | 187.09740 | 0.96 | 169.08130 ([M-H-OH]−) | Root/shoot |
| 125.09650 ([M-H-CHO2-OH]−) | |||||||||
| 41 | 12.47 | 284, 368 | Ferulic acid derivative IV | C20H29O10− | - | 429.17706 | - | 193.05023 ([ferulic acid]−) | Shoot |
| 179.03427 ([ferulic acid-CH3]−) | |||||||||
| 163.03958 ([ferulic acid-CH3-OH]−) | |||||||||
| 147.04450 ([ferulic acid-CH3-2OH]−) | |||||||||
| 42 | 12.70 | 259 | 2-Phenylethyl β-d-glucopyranoside | C14H19O6− | 283.11871 | 283.11893 | 0.78 | 267.12402 ([M-H-OH]−) | Root/shoot |
| 251.12881([M-H-2OH]−) | |||||||||
| 235.13390 ([M-H-3OH]−) | |||||||||
| 121.06506([M-H-C6H11O5]−) | |||||||||
| 43 | 12.75 | 272, 368 | Dalbergioidin | C15H11O6− | 287.05611 | 287.05618 | 0.24 | 271.06094 ([M-H-OH]−) | Shoot |
| 179.03467 ([M-H-C6H5O2]−) | |||||||||
| 165.05252 ([M-H-C6H5O3]−) | |||||||||
| 163.03951 ([M-H-C6H5O2-OH]−) | |||||||||
| 147.04404 ([M-H-C6H5O2-2OH]−) | |||||||||
| 125.02380 ([M-H-C9H9O3]−) | |||||||||
| 109.02868 ([M-H-C9H7O4]−) | |||||||||
| 44 | 12.95 | 260 | 4,8,12-trihydroxy-2,4-dodecadienoic acid, | C12H19O5− | 243.1238 | 243.12383 | 0.12 | 199.1337 ([M-H-CHO2]−) | Root |
| 139.11221 ([M-H-CHO2-C2H3-2OH]−) | |||||||||
| 45 | 13.40 | 283 | Caffeic acid isomer | C9H7O4− | 179.03498 | 179.03481 | 0.95 | 163.03954 ([M-H-OH]−) | Root |
| 135.04454 ([M-H-CHO2]−) | |||||||||
| 109.02881([M-H-C3H3O2]−) | |||||||||
| 46 | 13.80 | 222, 284 | β-d-Glucopyranoside, 1,1-dimethyl-5-methylenenonyl | C10H17O4− | 345.22826 | 345.22849 | 0.67 | 327.21780 ([M-H-OH]−) | Root |
| 315.21780 ([M-H-CH3O]−) | |||||||||
| 47 | 13.90 | 224, 284 | Sebacic acid | C10H17O4− | 201.11323 | 201.11293 | 1.49 | 185.11778 ([M-H-OH]−) | Shoot |
| 157.12276 ([M-H-CHO2]−) | |||||||||
| 48 | 13.93 | 223, 284 | alpha-Ionol O-[arabinosyl-(1->6)-glucoside] | C24H39O10− | 487.25487 | 487.25504 | 0.35 | 473.24008 ([M-H-CH3]−) | Shoot |
| 459.22311 ([M-H-2CH3]−) | |||||||||
| 355.21292 ([M-H-C5H9O4]−) | |||||||||
| 341.19687 ([M-H-C5H9O4-CH3]−) | |||||||||
| 49 | 15.35 | 283, 368, | Buteine | C15H11O5− | 271.0612 | 271.06131 | 0.41 | 163.03952 ([M-H-C6H5O2]−) | Root |
| 137.02380 ([M-H-C8H7O2]− | |||||||||
| 135.04443 ([M-H-C7H5O3]−) | |||||||||
| 121.02880 ([M-H-C8H7O2-OH]−) | |||||||||
| 108.02104 ([M-H-C9H7O3]−) | |||||||||
| 50 | 15.98 | 283 | D-xylofuranose tetradecyl glycoside | C22H41O9− | 449.27561 | 449.27576 | 0.33 | 403.27036 ([M-H-CH3O-OH]−) | Root |
| 316.22061 ([M-H-C5H9O4]−) | |||||||||
| 329.23349 ([M-H-C4H9-4OH]−) | |||||||||
| 117.05499 ([M-H-C17H32O5-OH]−) | |||||||||
| 51 | 16.42 | 283 | Corchorifatty acid F isomer | C18H31O5− | 327.21770 | 327.21799 | 0.89 | 309.20665 ([M-H-OH]−) | Root/shoot |
| 291.19684 ([M-H-2OH]−) | |||||||||
| 173.11787 ([M-H-C9H15O2]−) | |||||||||
| 157.12346 ([M-H-C9H15O2-OH]−) | |||||||||
| 125.09643([M-H-C3H5O2-C7H13O2]−) | |||||||||
| 52 | 18.30 | 283, 368 | Tianshic acid | C18H33O5− | 329.23335 | 329.23361 | 0.79 | 165.12788 ([M-H-C7H15O-3OH]−) | Root/shoot |
| 127.11205 ([M-H-C10H19O2-2OH]−) | |||||||||
| 53 | 19.15 | 283 | Dimethyl sebacate (sebacic acid derivative) | C12H21O4− | 229.14453 | 229.144 | 0.39 | 201.11287 ([sebacic acid]−) | Shoot |
| 215.12865 ([M-H-CH3]−) | |||||||||
| 211.13374 ([M-H-O]−) | |||||||||
| 199.13379 ([M-H-CH3O]−) | |||||||||
| 185.11778 ([M-H-CH3O-CH3]−) | |||||||||
| 157.12303 ([M-H-C2H3O2-CH3]−) | |||||||||
| 54 | 19.28 | 282, 368 | Unknown | C13H27O8− | - | 311.16888 | - | - | Root |
| 55 | 19.47 | 283, 368 | Corchorifatty acid F isomer | C18H31O5− | 327.21770 | 327.21802 | 0.98 | 309.20731 ([M-H-OH]−) | Root/shoot |
| 291.19672 ([M-H-2OH]−) | |||||||||
| 173.11792 ([M-H-C9H15O2]−) | |||||||||
| 125.09679 ([M-H-C3H5O2-C7H13O2]−) | |||||||||
| 56 | 20.06 | 283 | Unknown | C13H27O8− | - | 311.16888 | - | Shoot | |
| 57 | 20.63 | 283 | Nordihydrocapsiate | C17H25O4− | 293.17583 | 293.17612 | 0.99 | 277.18088 ([M-H-OH]−) | Root/shoot |
| 263.16534 ([M-H-CH3O]−) | |||||||||
| 247.16968 ([M-H-CH3O-OH]−) | |||||||||
| 157.12309 ([M-H-C8H9O2]−) | |||||||||
| 153.05524 ([M-H-C9H17O]−) | |||||||||
| 141.12810 ([M-H-C8H9O3]−) | |||||||||
| 58 | 20.92 | 274 | Plastoquinone 3 | C23H31O2− | 339.23296 | 339.23322 | 0.77 | 203.10753 ([M-H-C9H15]−) | Root |
| 163.11229 ([M-H-C6H11-C5H7O]−) | |||||||||
| 149.06009 ([M-H-C13H21]−) | |||||||||
| 135.04454 ([M-H-C14H23]−) | |||||||||
| 59 | 21.25 | 283 | Decyl gallate (gallic acid derivative) | C17H25O5− | 309.17075 | 309.17093 | 0.58 | 293.17935 ([M-H-OH]−) | Root/shoot |
| 169.01381 ([gallic acid]−) | |||||||||
| 153.01903([M-H-C10H21O]−) | |||||||||
| 125.02367 ([M-H-C11H21O2]−) | |||||||||
| 60 | 21.61 | 283 | Nordihydrocapsiate isomer | C17H25O4− | 293.17583 | 293.17612 | 0.99 | 277.18080 ([M-H-OH]−) | Root/shoot |
| 263.16535 ([M-H-CH3O]−) | |||||||||
| 247.16990 ([M-H-CH3O-OH]−) | |||||||||
| 157.12309 ([M-H-C8H9O2]−) | |||||||||
| 153.05524 ([M-H-C9H17O]−) | |||||||||
| 141.12810 ([M-H-C8H9O3]−) | |||||||||
| 61 | 22.30 | 283 | Unknown | C13H27O8− | - | 311.16904 | - | - | Root |
| 62 | 22.53 | 283 | Unknown | C24H45O11− | - | 509.29691 | - | - | Shoot |
| 63 | 23.36 | 283, 337 | 13-Hydroxyoctadecadienoic acid | C18H31O3− | 295.22787 | 295.22797 | 0.34 | 281.21204 ([M-H-CH3]−) | Root/shoot |
| 279.23334 ([M-H-OH]−) | |||||||||
| 169.12331 ([M-H-C8H15O]−) | |||||||||
| 153.12767 ([M-H-C8H15O-OH]−) | |||||||||
| 64 | 23.54 | 283, 337 | p-Hydroxynonanophenone | C15H21O2− | 233.1547 | 233.15462 | 0.34 | 219.17544 ([M-H-O]−) | Root/shoot |
| 167.14342 ([M-H-C4H5O]−) | |||||||||
| 135.04446 ([M-H-C7H15]−) | |||||||||
| 121.02875 ([M-H-C7H15-CH3]−) | |||||||||
| 65 | 24.03 | 283 | Unknown | C15H31O8− | - | 339.20029 | - | - | Root |
| 66 | 25.20 | 283 | Unknown | C14H29O8− | - | 325.18463 | - | - | Root |
| 67 | 25.72 | 283, 337 | Unknown | C14H29O8− | - | 325.18457 | - | - | Shoot |
| 68 | 25.98 | 283, 337 | Unknown | C14H29O8− | - | 325.18454 | - | - | Shoot |
| 69 | 26.24 | 283 | Unknown | C14H29O8− | - | 325.18463 | - | - | Root |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabañas-García, E.; Areche, C.; Jáuregui-Rincón, J.; Cruz-Sosa, F.; Pérez-Molphe Balch, E. Phytochemical Profiling of Coryphantha macromeris (Cactaceae) Growing in Greenhouse Conditions Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2019, 24, 705. https://doi.org/10.3390/molecules24040705
Cabañas-García E, Areche C, Jáuregui-Rincón J, Cruz-Sosa F, Pérez-Molphe Balch E. Phytochemical Profiling of Coryphantha macromeris (Cactaceae) Growing in Greenhouse Conditions Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules. 2019; 24(4):705. https://doi.org/10.3390/molecules24040705
Chicago/Turabian StyleCabañas-García, Emmanuel, Carlos Areche, Juan Jáuregui-Rincón, Francisco Cruz-Sosa, and Eugenio Pérez-Molphe Balch. 2019. "Phytochemical Profiling of Coryphantha macromeris (Cactaceae) Growing in Greenhouse Conditions Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry" Molecules 24, no. 4: 705. https://doi.org/10.3390/molecules24040705
APA StyleCabañas-García, E., Areche, C., Jáuregui-Rincón, J., Cruz-Sosa, F., & Pérez-Molphe Balch, E. (2019). Phytochemical Profiling of Coryphantha macromeris (Cactaceae) Growing in Greenhouse Conditions Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules, 24(4), 705. https://doi.org/10.3390/molecules24040705