Single-Piece Solid Contact Cu2+-Selective Electrodes Based on a Synthesized Macrocyclic Calix[4]arene Derivative as a Neutral Carrier Ionophore
Abstract
:1. Introduction
2. Results and Discussion
2.1. SWCNTs Versus PEDOT/PSS
2.2. Response Characteristics of the Solid-Contact Cu2+-ISEs
2.3. Water Film Test of the Electrode Potential
2.4. Sensors’ Applicability
3. Materials and Methods
3.1. Reagents
3.2. Apparatus
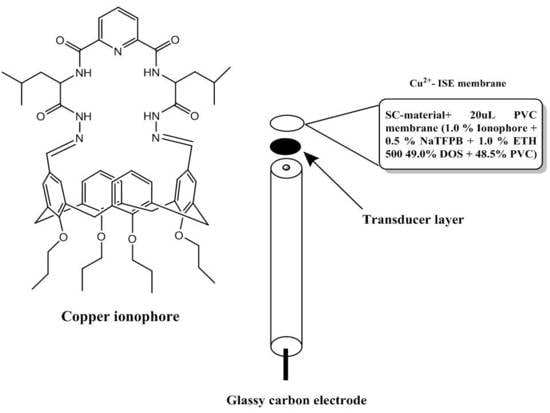
3.3. Electrode Fabrication
3.4. Analytical Application
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tyrala, E.E.; Brodsky, E.L.; Auerbach, V. Urinary copper losses in infants receiving free amino acid solutions. Am. J. Clin. Nutr. 1982, 35, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Narli, I.; Kiralp, S.; Toppare, L. Preventing inhibition of tyrosinase with modified electrodes. Anal. Chim. Acta 2006, 572, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bull, P.C.; Cox, D.W. Wilson disease and Menkes disease: New handles on heavy-metal transport. Trends Genet. 1994, 10, 246–252. [Google Scholar] [CrossRef]
- Schaefer, M.; Gitlin, G.D. Wilson’s disease and Menkes disease. Am. J. Physiol. 1999, 276, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.G.; Andrada, D.; Rey, U.V.; Ansaloni, L.M.S.; Borba da Silva, J.B. Employment of Ruthenium as Permanent Modifier for the Determination of Copper in Serum Samples by Electrothermal Atomic Absorption Spectrometry. Anal. Lett. 2006, 39, 2441–2451. [Google Scholar] [CrossRef]
- Cassella, R.J.; Magalhaes, O.I.B.; Couto, M.T.; Lima, E.L.S.; Neves, M.A.F.S.; Coutinho, F.M.B. Synthesis and application of a functionalized resin for flow injection/FAAS copper determination in waters. Talanta 2005, 67, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Queiroz, A.S.; Fernandes, M.S.; dos Santos, H.C. Application of factorial designs and Doehlert matrix in optimization of experimental variables associated with the preconcentration and determination of vanadium and copper in seawater by inductively coupled plasma optical emission spectrometry. Spectrochim. Acta Part B 2002, 57, 1939–1950. [Google Scholar] [CrossRef]
- Debrah, E.; Denoyer, E.R.; Tyson, J.F. Flow injection determination of mercury with preconcentration by amalgamation on a gold–platinum gauze by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1996, 11, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Mohadesi, A.; Taher, M.A. Voltammetric determination of Cu(II) in natural waters and human hair at a meso-2,3-dimercaptosuccinic acid self-assembled gold electrode. Talanta 2007, 72, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Shams, E.; Torabi, R. Determination of nanomolar concentrations of cadmium by anodic-stripping voltammetry at a carbon paste electrode modified with zirconium phosphated amorphous silica. Sens. Actuat. B 2006, 117, 86–92. [Google Scholar] [CrossRef]
- Ali, A.; Shen, H.; Yin, X. Simultaneous determination of trace amounts of nickel, copper and mercury by liquid chromatography coupled with flow-injection on-line derivatization and preconcentration. Anal. Chim. Acta 1998, 369, 215–223. [Google Scholar] [CrossRef]
- Harvey, D. Modern Analytical Chemistry; Wiley: New York, NY, USA, 2000; p. 816. [Google Scholar]
- Ivaska, A.; Kubiak, W.W. Application of sequential injection analysis to anodic stripping voltammetry. Talanta 1997, 44, 713–723. [Google Scholar] [CrossRef]
- Barreto, S.R.G.; Nozaki, J. Spectrophotometric Determination of Copper in Metallic Alloy Using a Bidithiolene: A Comparative Study. Microchem. J. 1999, 62, 223–228. [Google Scholar] [CrossRef]
- Chimpalee, N.; Chimpalee, D.; Lohwithee, S.; Nakwatchara, L.; Burns, D.T. Spectrophotometric determination of copper after extraction of its chelate with bis(acetylacetone)ethylenediimine. Anal. Chim. Acta 1996, 329, 315–318. [Google Scholar] [CrossRef]
- Karabocek, S.; Nohut, S.; Dalman, O.; Guner, S. A new spectrophotometric reagent for copper: 3,3′-(1,3-propanediyldiimine) bis-[3-methyl-2-butanone]dioxime. Anal. Chim. Acta 2000, 408, 163–168. [Google Scholar] [CrossRef]
- Van Staden, J.F.; Botha, A. Spectrophotometric determination of Cu (II) with sequential injection analysis. Talanta 1999, 49, 1099–1108. [Google Scholar] [CrossRef]
- Neudachina, L.K.; Oshintseva, E.V.; Skorik, Y.A.; Vshivkova, A.A. N-Aryl-3-Aminopropionic acids as selective reagents for the determination of copper in metallurgical products. J. Anal. Chem. 2005, 60, 240–246. [Google Scholar] [CrossRef]
- Kamel, A.H.; Mahmoud, W.H.; Mostafa, M.S. Response Characteristics of Copper-Selective Polymer Membrane Electrodes Based on a Newly Synthesized Macrocyclic Calix[4]arene Derivative as a Neutral Carrier Ionophore. Electroanalysis 2010, 22, 2453–2459. [Google Scholar] [CrossRef]
- Kamel, A.H.; Kalifa, M.E.; Abd El-Maksoud, S.A.; Elgendy, F.A. Fabrication of novel sensors based on a synthesized acyclic pyridine derivative ionophore for potentiometric monitoring of copper. Anal. Methods 2014, 6, 7814–7822. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Elnemma, E.M.; Kamel, A.H. Novel potentiometric copper (II) selective membrane sensors based on cyclic tetrapeptide derivatives as neutral ionophores. Talanta 2015, 66, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Galal, H.R.; Awwad, N.S. Cost-effective and handmade paper-based potentiometric sensing platform for piperidine determination. Anal. Methods 2018, 10, 5406–5415. [Google Scholar] [CrossRef]
- Crespo, G.A. Recent advances in ion-selective membrane electrodes for in situ environmental water analysis. Electrochim. Acta 2017, 245, 1023–1034. [Google Scholar] [CrossRef]
- Van de Velde, L.; Angremont, E.; Olthuis, W. Solid contact potassium selective electrodes for biomedical applications-a review. Talanta 2016, 160, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, C.; Diamond, D. Opportunities and challenges of using ion-selective electrodes in environmental monitoring and wearable sensors. Electrochim. Acta 2012, 84, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Kamel, A.H.; Almedia, S.A.A.; Sales, M.G.F.; Moreira, F.T.C. Sulfadiazine-Potentiometric Sensors for Flow and Batch Determinations of Sulfadiazine in Drugs and Biological Fluids. Anal. Sci. 2009, 25, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalska, A. Optimizing the analytical performance and construction of ion-selective electrodes with conducting polymer-based ion-to-electron transducers. Anal. Bioanal. Chem. 2006, 384, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J. Conducting polymer-based solid-state ion-selective electrodes. Electroanalysis 2010, 18, 7–18. [Google Scholar] [CrossRef]
- Hernández, R.; Riu, J.; Bobacka, J.; Vallés, C.; Jiménez, P.; Benito, A.M.; Maser, W.K.; Rius, F.X. Reduced graphene oxide films as solid transducers in potentiometric all solid-state ion-selective electrodes. J. Phys. Chem. C 2012, 116, 22570–22578. [Google Scholar] [CrossRef]
- Ye, J.; Li, F.; Gan, S.; Jiang, Y.; An, Q.; Zhang, Q.; Niu, L. Using sp2-C dominant porous carbon sub-micrometer spheres as solid transducers in ion-selective electrodes. Electrochem. Commun. 2015, 50, 60–63. [Google Scholar]
- Rius-Ruiz, F.X.; Crespo, G.A.; Bejarano-Nosas, D.; Blondeau, P.; Riu, J.; Rius, F.X. Potentiometric strip cell based on carbon nanotubes as transducer layer: Toward low-cost decentralized measurements. Anal. Chem. 2011, 83, 8810–8815. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon nanotube-based ion selective sensors for wearable applications. ACS Appl. Mater. Interface 2017, 9, 35169–35177. [Google Scholar] [CrossRef] [PubMed]
- Wardak, C.; Lenik, J. Application of ionic liquid to the construction of Cu(II) ion-selective electrode with solid contact. Sens. Actuat. B 2013, 189, 52–59. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, C.; Wang, R.; Hu, G.; Miaoc, J.; Haia, K.; Lin, C. Determination of copper(II) ion in food using an ionic liquids-carbon nanotubes-based ion-selective electrode. J. Food Comp. Anal. 2017, 62, 63–68. [Google Scholar] [CrossRef]
- Ganjali1, M.R.; Aghabalazadeh, S.; Khoobi, M.; Ramazani, A.; Foroumadi, A.; Shafiee, A.; Norouzi, P. Nanocomposite Based Carbon Paste Electrode for Selective Analysis of Copper. Int. J. Electrochem. Sci. 2011, 6, 52–62. [Google Scholar]
- Liang, R.; Yin, T.; Qin, W. A simple approach for fabricating solid-contact ion-selective electrodes using nanomaterials as transducers. Anal. Chim. Acta 2015, 853, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pięk, M.; Fendrych, K.; Smajdor, J.; Piech, R.; Paczosa-Bator, B. High selective potentiometric sensor for determination of nanomolar concentration of Cu(II) using a polymeric electrode modified by a graphene/7,7,8,8 tetracyanoquinodimethane nanoparticles. Talanta 2017, 170, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Mattinen, U.; Rabiej, S.; Lewenstam, A.; Bobacka, J. Impedance study of the ion-to-electron transduction process for carbon cloth as solid-contact material in potentiometric ion sensors. Electrochim. Acta 2011, 56, 10683–10687. [Google Scholar] [CrossRef]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]
- Brinić, S.; Buzuk, M.; Bralić, M.; Generalić, E. Solid-contact Cu(II) ion-selective electrode based on 1,2-di-(o-salicylaldiminophenylthio) ethane. J. Solid State Electrochem. 2012, 16, 1333–1341. [Google Scholar] [CrossRef]
- Andac, M.; Coldur, F.; Bilir, S.; Birinci, A.; Demir, S.; Uzun, H. Solid-contact polyvinyl chloride membrane electrode based on the bis[(2(hydroxyethylimino) phenolato] copper(II) complex for trace level determination of copper ions in wastewater. Can. J. Chem. 2014, 92, 324–328. [Google Scholar] [CrossRef]
- Woznica, E.; Wojcik, M.M.; Mieczkowski, J.; Maksymiuk, K.; Michalska, A. Dithizone Modified Gold Nanoparticles Films as Solid Contact for Cu2+ Ion-Selective Electrodes. Electroanalysis 2013, 25, 141–146. [Google Scholar] [CrossRef]
- Birinci, A.; Eren, H.; Coldur, F.; Coskun, E.; Andac, M. Rapid determination of trace level copper in tea infusion samples by solid contact ion selective electrode. J. Food Drug Anal. 2016, 24, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amr, A.E.; Mohamed, A.M.; Ibrahim, A.A. Synthesis of some new chiral tricyclic and macrocyclic pyridine derivatives as antimicrobial agents. Z. Naturfosch. 2003, 58b, 861. [Google Scholar] [CrossRef]
Sample Availability: Not available. |




| Parameter | GC/Cu2+-ISE (CWE) | GC/SWCNTs/Cu2+-ISE | GC/PEDOT/PSS/Cu2+-ISE |
|---|---|---|---|
| Slope (mV/decade) | 28.0 ± 0.4 | 27.8 ± 0.3 | 28.1 ± 0.4 |
| Correlation coefficient (r2) | 0.9993 | 0.9997 | 0.9990 |
| Detection limit (M) | 5.0 × 10−10 | 5.4 × 10−10 | 5.0 × 10−10 |
| Linear range (M) | 1.0 × 10−9–1.0 × 10−2 | 2.0 × 10−9–1.0 × 10−2 | 1.0 × 10−9–1.0 × 10−2 |
| Response time (s) | <5 | <5 | <5 |
| Working pH range (pH) | 3.0–6.5 | 3.0–6.5 | 3.0–6.5 |
| Accuracy (mV%) | 99.6 | 99.3 | 98.8 |
| Precision (mV%) | 1.1 | 1.2 | 1.7 |
| Between-day variability (mV%) | 0.9 | 1.5 | 1.3 |
| Interfering Ion, J | log KCu, J ± SD | ||
|---|---|---|---|
| GC/Cu2+-ISE | GC/SWCNTs-Cu2+-ISE | GC/PEDOT/PSS/Cu2+-ISE | |
| Na+ | −6.5 ± 0.3 | −6.6 ± 0.4 | −6.3 ± 0.3 |
| Mg2+ | −8.4 ± 0.1 | −8.5 ± 0.3 | −8.4 ± 0.4 |
| Ca2+ | −8.8 ± 0.2 | −8.7 ± 0.1 | −8.6 ± 0.3 |
| K+ | −5.1 ± 0.7 | −5.0 ± 0.6 | −5.0 ± 0.5 |
| Pb2+ | −4.5 ± 0.8 | −4.3 ± 0.4 | −4.4 ± 0.1 |
| Cd2+ | −7.6 ± 0.2 | −7.6 ± 0.3 | −7.4 ± 0.4 |
| Zn2+ | −7.2 ± 0.3 | −7.3 ± 0.1 | −7.4 ± 0.2 |
| Ni2+ | −8.1 ± 0.7 | −8.2 ± 0.4 | −8.0 ± 0.3 |
| Ag+ | −3.9 ± 0.4 | −3.7 ± 0.7 | −3.8 ± 0.4 |
| Hg2+ | −4.1 ± 0.5 | −4.2 ± 0.3 | −4.1 ± 0.4 |
| Sample | (µg/mL) * | Recovery (%) | |||
|---|---|---|---|---|---|
| Proposed Sensor | AAS | Added | Found | ||
| Sample 1 | 1.10 ± 0.2 | 1.05 ± 0.3 | 0.3 | 1.42 ± 0.1 | 106.6 |
| 0.5 | 1.62 ± 0.2 | 104.0 | |||
| 0.8 | 1.87 ± 0.3 | 96.2 | |||
| Sample 2 | 0.80 ± 0.07 | 0.82 ± 0.04 | 0.3 | 1.08 ± 0.4 | 93.3 |
| 0.5 | 1.31 ± 0.2 | 102.0 | |||
| 0.8 | 1.58 ± 0.1 | 97.5 | |||
| Tea Samples | Copper Content ± SD (mg/Kg) a | t-Test b | |
|---|---|---|---|
| Potentiometry | AAS | ||
| Lipton (Black Sri Lankan Tea, Cairo, Egypt) | 8.3 ± 0.7 | 8.0 ± 0.3 | 2.37 |
| Ahmed Tea (Black Tea, London, UK) | 12.3 ± 0.5 | 12.6 ± 0.4 | 2.61 |
| Al-Arosa (Dust Black Kenyan tea, Cairo, Egypt) | 23.4 ± 0.8 | 22.8 ± 0.2 | 2.53 |
| Al-Rabea (Black Tea, Riyadh, Saudi Arabia) | 21.3 ± 0.6 | 22.1 ± 0.3 | 2.67 |
| Dilmah (Sri Lankan Tea, London, UK) | 17.3 ± 0.8 | 16.7 ± 0.1 | 2.43 |
| Ionophore | Solid Contact Material | Slope, mV/Decade | Detection Limit, M | Linear Range, M | Potential Drift, µV/s | Capacitance, µF | Selectivity Coefficients, log KCu2+, J, Method Used | Ref. |
|---|---|---|---|---|---|---|---|---|
| o-Xylylene bis (N,N-diisobutyldithiocarbamate | Graphite | 31.3 | 4.9 × 10−7 | 1.0 × 10−6–1.0 × 10−2 | NR | NR | Co2+ (−3.8), Na+ (−4.7), K+ (−2.4), Zn2+ (−5.2), Ba2+ (−4.5), NH4+ (−4.1), Ni2+ (−2.3), Cd2+ (−3.0), Ca2+ (−3.5), Pb2+ (−2.5). SSM | [44] |
| N,N,N′,N′-Tetradodecyl-3,6-dioxaoctanedithioamide | SWCNTs | 29.8 | 4.0 × 10−9 | 1.0 × 10−4–1.0 × 10−8 | 5.2 | Na+ (−10.5), K+ (−8.6), Ca2+ (−11.9), Mg2+ (−13.3). SSM | [36] | |
| N,N,N′,N′-Tetracyclohexyl-2,2′-thiodiacetamide | 1-Ethyl-3-methyl imidazolium chloride. | 28.9 | 3.2 × 10−8 | 1.0 × 10−7–1.0 × 10−1 | NR | NR | Co2+ (−3.16), Na+ (−4.95), K+ (−5.21), Zn2+ (−3.39), Mg2+ (−6.22), Li+ (−5.11), Ni2+ (−3.02), Cd2+ (−3.84), Ca2+ (−4.93). SSM | [33] |
| 1-(2-Aminoethyl)-3-butyl imidazolium bis (trifluoromethane sulfonyl) imide | Carboxylic multi-walled carbon nanotubes (MWCNTs-COOH) | 8.17 | 7.9 × 10−11 | 1.0 × 10-10–1.0 × 10−5 | NR | NR | Co2+ (−2.7), Na+ (−4.1), K+ (−3.9), Zn2+ (−2.5), Mg2+ (−3.4), NH4+ (−3.5), Ni2+ (−3.0), Mn2+ (−3.7), Ca2+ (−3.0), Pb2+ (−3.1), Cr3+ (−3.3), Fe3+ (−3.3). FIM | [34] |
| (bis-[(2-(Hydroxyethylimino) phenolato]copper(II)) | NR | 28.3 | 8.3 × 10−7 | 1.0 × 10−6−1.0 × 10−1 | NR | NR | Na+ (−4.3), K+ (−4.3), Ca2+ (−4.4), Ba2+ (−4.5), Pb2+ (−3.8), Zn2+ (−3.0), Co2+ (−3.1), Ni2+ (−3.2), Cd2+ (−4.6), Cr3+ (−1.6). SSM | [42] |
| 3-(2-Methyl-2,3-dihydrobenzothiazol-2-yl)-2H-chromen-2-one | MWCNTs | 29.3 | 7.9 × 10−7 | 1.0 × 10−6−1.0 × 10−1 | NR | NR | Hg2+ (−2.3), Lu3+ (−2.5), K+ (−3.1), Zn2+ (−3.9), Gd2+ (−3.8), Ag+ (−3.7), Ni2+ (−3.8), Mn2+ (−2.8), Cd2+ (−3.8), Ca2+ (−3.1), Pb2+ (−3.5), Cr3+ (−2.3), Fe3+ (−2.8), La3+ (−3.2). MPM | [35] |
| 7,7,8,8-Tetracyanoquinodimethane | Graphen (GR) Graphenoxide (GO) | 30.5 30.6 | 1.0 × 10−9.2 1.0 × 10−7.5 | 1.0 × 10−9–1.0 × 10−2 1.0 × 10−7–1.0 × 10−2 | 20.2 54.5 | 495 183 | K+ (−5.02), Na+ (−5.26), Ag+ (3.40), Mg2+ (−5.89), Ca2+ (−5.06), Zn2+ (−2.49), Pb2+ (−1.88), Ni2+ (−2.52). SSM K+ (−5.52), Na+ (−6.27), Ag+ (3.42), Mg2+ (−6.84), Ca2+ (−5.76), Zn2+ (−2.94), Pb2+ (−2.76), Ni2+ (−2.97). SSM | [37] |
| Dithizone | Gold nanoparticle | 23.5 24.1 22.1 | 1.0 × 10−5.5 1.0 × 10−6 1.0 × 10−7.5 | 1.0 × 10−5–1.0 × 10−1 1.0 × 10−5–1.0 × 10−1 1.0 × 10−7–1.0 × 10−1 | NR NR NR | 11 14 13 | K+ (−3.8), Na+ (−6.2), Mg2+ (−8.2), Ca2+ (−8.7), Zn2+ (−7.1), Pb2+ (−3.2), Ni2+ (−8.1), Cd2+ (−6.0). SSM K+ (−3.0), Na+ (−5.2), Mg2+ (−7.3), Ca2+ (−7.8), Zn2+ (−6.3), Pb2+ (−3.1), Ni2+ (−7.2), Cd2+ (−5.4). SSM K+ (−2.3), Na+ (−2.9), Mg2+ (−4.4), Ca2+ (−6.1), Zn2+ (−4.1), Pb2+ (−1.7), Ni2+ (−4.5), Cd2+ (−4.5). SSM | [43] |
| 1,2-di-(o-Salicylaldimino -phenylthio) ethane | Carbon ink | 31.0 | 1.6 × 10−6 | 3.2 × 10−6–2.8 × 10−2 | NR | NR | K+ (−7.0), Na+ (−7.0), Mn2+ (−2.6), Ca2+ (−7.0), Zn2+ (−2.3), Pb2+ (−2.0), Ni2+ (−7.0), Cd2+ (−4.1), Ag+ (−7.0), Co2+ (−5.0), Fe2+ (−5.0). MPM | [41] |
| Macrocyclic calix[4]arene derivative | SWCNTs PEDOT/PSS | 27.8 ± 0.3 28.1 ± 0.4 | 5.4 × 10−10 5.0 × 10−10 | 1.0 × 10−3–2.0 × 10−9 1.0 × 10−3–1.0 × 10−9 | 30.1 ± 2.5 16.6 ± 1.2 | 33.3 ± 1.3 60.2 ± 0.2 | Mg2+ (−8.5), Na+ (−6.6), K+ (−5.0), Zn2+ (−7.3), Hg2+ (−4.2), Ag+ (−3.7), Ni2+ (−8.2), Cd2+ (−7.6), Ca2+ (−8.7), Pb2+ (−4.3). SSM Mg2+ (−8.4), Na+ (−6.3), K+ (−5.0), Zn2+ (−7.4), Hg2+ (−4.1), Ag+ (−3.8), Ni2+ (−8.0), Cd2+ (−7.4), Ca2+ (−8.6), Pb2+ (−4.4). SSM | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
E. Amr, A.E.-G.; Al-Omar, M.A.; H. Kamel, A.; A. Elsayed, E. Single-Piece Solid Contact Cu2+-Selective Electrodes Based on a Synthesized Macrocyclic Calix[4]arene Derivative as a Neutral Carrier Ionophore. Molecules 2019, 24, 920. https://doi.org/10.3390/molecules24050920
E. Amr AE-G, Al-Omar MA, H. Kamel A, A. Elsayed E. Single-Piece Solid Contact Cu2+-Selective Electrodes Based on a Synthesized Macrocyclic Calix[4]arene Derivative as a Neutral Carrier Ionophore. Molecules. 2019; 24(5):920. https://doi.org/10.3390/molecules24050920
Chicago/Turabian StyleE. Amr, Abd El-Galil, Mohamed A. Al-Omar, Ayman H. Kamel, and Elsayed A. Elsayed. 2019. "Single-Piece Solid Contact Cu2+-Selective Electrodes Based on a Synthesized Macrocyclic Calix[4]arene Derivative as a Neutral Carrier Ionophore" Molecules 24, no. 5: 920. https://doi.org/10.3390/molecules24050920
APA StyleE. Amr, A. E. -G., Al-Omar, M. A., H. Kamel, A., & A. Elsayed, E. (2019). Single-Piece Solid Contact Cu2+-Selective Electrodes Based on a Synthesized Macrocyclic Calix[4]arene Derivative as a Neutral Carrier Ionophore. Molecules, 24(5), 920. https://doi.org/10.3390/molecules24050920