Triterpene Acid and Phenolics from Ancient Apples of Friuli Venezia Giulia as Nutraceutical Ingredients: LC-MS Study and In Vitro Activities
Abstract
:1. Introduction
2. Results
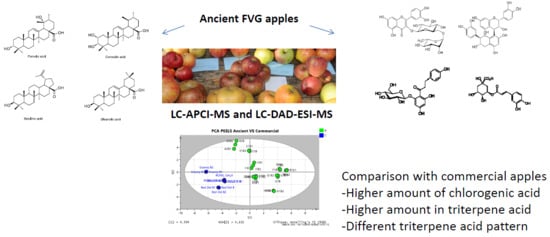
2.1. Secondary Metabolite Fingerprinting of Ancient Apples
2.2. Multivariate Analysis
2.3. Spectrophotometric Assays to Assess Total Phenolic and Flavonoid Contents
2.4. Pulp Composition
2.5. Peels Composition
2.6. Biological Activities
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sampling
4.2. Chemicals and Instruments
4.3. Sample Preparation
4.4. HPLC-DAD-(ESI)-MS Analysis
4.5. HPLC-(APCI)-MS Analysis
4.6. Multivariate Analysis
4.7. Total Bioactive Compounds
4.8. Assays for Enzyme Inhibition and Antioxidant Capacity
4.9. Statistical Evaluation for Total Bioactive Compounds and Biological Activity Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis. BMJ 2014, 4490, 1–14. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-siwkiewicz, H.; Priebe, W. Plant Physiology and Biochemistry Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kevser, S.; Amasya, S.; Spur, S.; Spur, E.; Luscious, K.; Kizi, A.; Golden, L. Comparison of antioxidant capacity and phenolic composition of peel and flesh of some apple varieties, eyda Karaman, a Esma T utem. J. Sci. Food Agric. 2013, 93, 867–875. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hortic. 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef]
- Belviso, S.; Scursatone, B.; Re, G.; Zeppa, G.; Belviso, S.; Scursatone, B.; Re, G.; Zeppa, G.; Belviso, S.; Scursatone, B.; et al. Novel Data on the Polyphenol Composition of Italian Ancient Apple Cultivars. Int. J. Food Prop. 2013, 16, 1507–1515. [Google Scholar] [CrossRef] [Green Version]
- Awad, M.A.; De Jager, A.; Westing, L.M. Van Flavonoid and chlorogenic acid levels in apple fruit: Characterisation of variation. Sci. Hortic. 2000, 83, 249–263. [Google Scholar] [CrossRef]
- Carbone, K.; Giannini, B.; Picchi, V.; Scalzo, R.L.; Cecchini, F. Phenolic composition and free radical scavenging activity of different apple varieties in relation to the cultivar, tissue type and storage. Food Chem. 2011, 127, 493–500. [Google Scholar] [CrossRef]
- Askowski, P.I.L. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar]
- Sut, S.; Baldan, V.; Faggian, M.; Peron, G.; Dall’Acqua, S. Nutraceuticals, A new challenge for medicinal chemistry. Curr. Med. Chem. 2016, 23, 3198–3223. [Google Scholar] [CrossRef]
- Joy, J.M.; Vogel, R.M.; Moon, J.R.; Falcone, P.H.; Mosman, M.M.; Pietrzkowski, Z.; Reyes, T.; Kim, M.P. Ancient peat and apple extracts supplementation may improve strength and power adaptations in resistance trained men. BMC Complement. Altern. Med. 2016, 1–9. [Google Scholar] [CrossRef]
- Andre, C.M.; Greenwood, M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Perry, N.B.; Laing, W.A. Anti-In fl ammatory Procyanidins and Triterpenes in 109 Apple Varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Carlo, G.; Campiglia, P.; Stiuso, P.; Ritieni, A.; Novellino, E. Nutraceutical potential of polyphenolic fractions from Annurca apple (M. pumila Miller cv Annurca). Food Chem. 2013, 140, 614–622. [Google Scholar] [CrossRef]
- Panzella, L.; Petriccione, M.; Rega, P.; Scortichini, M.; Napolitano, A. A reappraisal of traditional apple cultivars from Southern Italy as a rich source of phenols with superior antioxidant activity. Food Chem. 2013, 140, 672–679. [Google Scholar] [CrossRef]
- Carlo, G.; Calabrese, G.; Stiuso, P.; Ritieni, A.; Giannetti, D.; Novellino, E. Effects of Annurca apple polyphenols on lipid metabolism in HepG2 cell lines: A source of nutraceuticals potentially indicated for the metabolic syndrome. FRIN 2014, 63, 252–257. [Google Scholar] [CrossRef]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the In Vitro Antioxidant Capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef]
- Morresi, C.; Cianfruglia, L.; Armeni, T.; Mancini, F.; Carlo, G.; Emanuela, T.; Ambra, D.U.; Gianna, M.; Bacchetti, T. Polyphenolic compounds and nutraceutical properties of old and new apple cultivars. J. Food Biochem. 2018, 42, 1–11. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; Avino, M.D.; Santamaria, R.; Irace, C.; Piccolo, M.; Maisto, M.; Novellino, E. Annurca Apple Nutraceutical Formulation Enhances Keratin Expression in a Human Model of Skin and Promotes Hair Growth and Tropism in a Randomized Clinical Trial. J. Med. Food 2018, 21, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Pagano, F.; Ostacolo, C.; Carlo, G.; Teresa, M.; Novellino, E.; Manfra, M.; Campiglia, P. Detailed polyphenolic pro fi ling of Annurca apple (M. pumila Miller cv Annurca) by a combination of RP-UHPLC and HILIC, both hyphenated to IT-TOF mass spectrometry. FRIN 2015, 76, 466–477. [Google Scholar] [CrossRef]
- Mari, A.; Tedesco, I.; Nappo, A.; Luigi, G.; Malorni, A.; Carbone, V. Phenolic compound characterisation and antiproliferative activity of “Annurca” apple, a southern Italian cultivar. Food Chem. 2010, 123, 157–164. [Google Scholar] [CrossRef]
- Tenore, G.; Carotenuto, A.; Caruso, D.; Buonomo, G.; Avino, M.D.; Brancaccio, D.; Ciampaglia, R.; Maisto, M.; Schisano, C.; Novellino, E. A nutraceutical formulation based on Annurca apple polyphenolic extract is e ff ective on intestinal cholesterol absorption: A randomised, placebo- controlled, crossover study. PharmaNutrition 2018, 6, 85–94. [Google Scholar] [CrossRef]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S. Potent Anti-Inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated through Suppression of NF-kB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef]
- Gaje, M.; Michalczuk, L.; Koziołkiewicz, M.; Babuchowski, A.; Zielonka, Ł.; Lewczuk, B. The influence of a natural triterpene preparation on the gastrointestinal tract of gilts with streptozocin-induced diabetes and on cell metabolic activity. J. Funct. Foods 2017, 33, 11–20. [Google Scholar] [CrossRef]
- Silva, F.S.G.; Oliveira, P.J.; Duarte, M.F. Oleanolic, Ursolic, and Betulinic Acids as Food Supplements or Pharmaceutical Agents for Type 2 Diabetes: Promise or Illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef]
- Jakobek, L.; Barron, A.R. Ancient apple varieties from Croatia as a source of bioactive polyphenolic compounds. J. Food Compos. Anal. 2016, 45, 9–15. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viškelis, P.; Jakštas, V.; Raudonis, R.; Kviklys, D.; Milašius, A.; Janulis, V. Application of an Optimized HPLC Method for the Detection of Various Phenolic Compounds in Apples from Lithuanian Cultivars. J. Chem. 2014, 2014, 542121. [Google Scholar] [CrossRef]
- Giannetti, V.; Boccacci, M.; Mannino, P.; Marini, F. Volatile fraction analysis by HS-SPME/GC-MS and chemometric modeling for traceability of apples cultivated in the Northeast Italy. Food Control 2017, 78, 215–221. [Google Scholar] [CrossRef]
- Abrosca, B.D.; Scognamiglio, M.; Corrado, L.; Chiocchio, I.; Zampella, L.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Fiorentino, A.; Petriccione, M. Evaluation of different training systems on Annurca apple fruits revealed by agronomical, qualitative and NMR-based metabolomic approaches. Food Chem. 2017, 222, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Poloniato, G.; Malagoli, M.; Dall’acqua, S. Fragmentation of the main triterpene acids of apple by LC- APCI-MSn. J. Mass Spectrom. 2018, 53, 882–892. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; Luca, E.D.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Atere, T.G.; Akinloye, O.A.; Ugbaja, R.N.; Ojo, D.A.; Dealtry, G. In vitro antioxidant capacity and free radical scavenging evaluation of standardized extract of Costus afer leaf. Food Sci. Hum. Wellness 2018, 7, 266–272. [Google Scholar] [CrossRef]
- Athipornchai, A.; Jullapo, N. Tyrosinase inhibitory and antioxidant activities of Orchid (Dendrobium spp.). S. Afr. J. Bot. 2018, 119, 188–192. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic characterization of aging wine lees: Correlation with antioxidant activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef]
- Jiao, Y.; Kilmartin, P.A.; Fan, M.; Quek, S.Y. Assessment of phenolic contributors to antioxidant activity of new kiwifruit cultivars using cyclic voltammetry combined with HPLC. Food Chem. 2018, 268, 77–85. [Google Scholar] [CrossRef]
- Vargas-León, E.A.; Díaz-Batalla, L.; González-Cruz, L.; Bernardino-Nicanor, A.; Castro-Rosas, J.; Reynoso-Camacho, R.; Gómez-Aldapa, C.A. Effects of acid hydrolysis on the free radical scavenging capacity and inhibitory activity of the angiotensin converting enzyme of phenolic compounds of two varieties of jamaica (Hibiscus sabdariffa). Ind. Crops Prod. 2018, 116, 201–208. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Phenolic acids contribution to antioxidant activities and comparative assessment of phenolic content in mango pulp and peel. S. Afr. J. Bot. 2018, 116, 158–163. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Yanni, A.E.; Koutrotsios, G.; Aloupi, M. Bioactive microconstituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece. Food Chem. Toxicol. 2013, 55, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, X.; Chen, Q.; Lu, J.; Rapposelli, S.; Pi, R. A review on the hybrids of hydroxycinnamic acid as multi-target-directed ligands against Alzheimer’s disease. Bioorg. Med. Chem. 2018, 26, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ferruzzi, M.G.; Ho, L.; Blount, J.; Janle, E.M.; Gong, B.; Pan, Y.; Gowda, G.A.N.; Raftery, D.; Arrieta-Cruz, I. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J. Neurosci. 2012, 32, 5144–5150. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, X.; Huang, L.; Gong, H.; Cheng, B.; Sun, Y.; Li, Y.; Liu, Q.; Zheng, L.; Huang, K. Proanthocyanidins are the major anti-diabetic components of cinnamon water extract. Food Chem. Toxicol. 2013, 56, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J.; Borzym-Kluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Ruszkowski, P.; Bobkiewicz-Kozlowska, T. Natural triterpenoids and their derivatives with pharmacological activity against neurodegenerative disorders. Mini-Rev. Org. Chem. 2014, 11, 307–315. [Google Scholar] [CrossRef]
- Chen, C.; Lim, Y. Receptor to Attenuate Ligand-Induced Lipogenesis. J. Agric. Food Chem. 2018, 66, 10964–10976. [Google Scholar] [CrossRef]
- Yap, W.H.; Lim, Y.M. Mechanistic Perspectives of Maslinic Acid in Targeting Inflammation. Biochem. Res. Int. 2015, 2015, 279356. [Google Scholar] [CrossRef]
- Fukumitsu, S.; Villareal, M.O.; Fujitsuka, T.; Aida, K. Anti-inflammatory and anti-arthritic effects of pentacyclic triterpenoids maslinic acid through NF-κB inactivation. Mol. Nutr. Food Res. 2016, 60, 399–409. [Google Scholar] [CrossRef]
- Jeszka, M.; Aleksandra, S.; Krystyna, S. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
Sample Availability: Samples of the plant tissues are available from the authors. |










| n. | RT (min) | UV (nm) | [M − H]− | MS2 | MS3 and MS4 | Compound |
|---|---|---|---|---|---|---|
| 1 | 5 | 280 | 289 | 245–205–179 | catechin * | |
| 2 | 6.4 | 330 | 353 | 191–179 | 173–171–127–85 | chlorogenic acid * |
| 3 | 6.5 and 6.8 | 280 | 577 | 451–425–407–289 | 407–381 | procyanidin dimer B1 * |
| 4 | 6.9 | 330 | 387 | 341–179–161–143 | caffeic acid derivative | |
| 5 | 9.8 | 280 | 865 | 739–713–695–577–425–407 | procyanidin trimer B | |
| 6 | 11.1 | 280 | 1441 | 1151–865–577 | procyanidin pentamer B | |
| 7 | 11.9 | 280 | 1153 | 1027–983–865–577–575 | procyanidin tetramer B | |
| 8 | 14.9 | 280 | 583 | 289 | flavan-3-ol derivative | |
| 9 | 15 | 350 | 463 | 301 | 271–255–179 | Quercetin-3-O-galactoside * |
| 10 | 15.5 | 350 | 463 | 301 | 271–255–179 | quercetin-3-O-glucoside * |
| 11 | 15.5 | 350 | 609 | 301 | 271–255–179 | rutin * |
| 12 | 16.1 | 350 | 433 | 301 | 271–255–179 | quercetin-3-O-xyloside * |
| 13 | 16.5 | 280 | 567 | 273 | 167 | phloretin-2-O-xyloglucoside * |
| 14 | 16.9 | 350 | 433 | 301 | 271–255–179 | quercetin-3-O-arabynoside * |
| 15 | 17.2 | 350 | 447 | 301 | 271–255–179 | quercetin-3-O-rhamnoside * |
| 16 | 18.2 | 280 | 435 | 273 | 167–123 | phloretin-2-O-glucoside * |
| 17 | 18.5 | 350 | 477 | 315–285–274 | rhamnetin-3-O-glucoside * | |
| 18 | 25.5 | ns | 501.5 | 471.6–453.6–427.6–409.6 | cuneataol | |
| 19 | 26.1 | ns | 501.5 | 483.5–457.6–441.5–409.7 | 379.5 | pomaceic acid * |
| 20 | 27.1 | ns | 487.5 | 469.5–425.6–407.6 | 405.6–393.6 | euscaphyc acid * |
| 21 | 28 | ns | 485.6 | 467.5–441.6–423.6–405.7 | 405.7–393.6 | annurcoic acid * |
| 22 | 28.5 | ns | 471.6 | 453.6–411.6–407.6 | 393.5–391.5 | pomolic acid * |
| 23 | 28.6 | ns | 471.5 | 423.5–405.6–393.5–377.6 | maslinic acid * | |
| 24 | 28.7 | ns | 471.5 | 451.6–423.5–405.6–393.5 | 407.5–405.6–393.5 | corosolic acid * |
| 25 | 29.5 | ns | 469.5 | 423.6–405.5–393.6 | euscaphic acid derivative | |
| 26 | 30.5 | ns | 455.5 | 414.7–409.7–393.6 | betulinic acid * | |
| 27 | 33 | ns | 455.5 | 407 | 391.5–378.6–365.5 | oleanolic acid * |
| 28 | 33 | ns | 455.5 | 407 | 391.5–378.6–365.5 | ursolic acid * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sut, S.; Zengin, G.; Maggi, F.; Malagoli, M.; Dall’Acqua, S. Triterpene Acid and Phenolics from Ancient Apples of Friuli Venezia Giulia as Nutraceutical Ingredients: LC-MS Study and In Vitro Activities. Molecules 2019, 24, 1109. https://doi.org/10.3390/molecules24061109
Sut S, Zengin G, Maggi F, Malagoli M, Dall’Acqua S. Triterpene Acid and Phenolics from Ancient Apples of Friuli Venezia Giulia as Nutraceutical Ingredients: LC-MS Study and In Vitro Activities. Molecules. 2019; 24(6):1109. https://doi.org/10.3390/molecules24061109
Chicago/Turabian StyleSut, Stefania, Gokhan Zengin, Filippo Maggi, Mario Malagoli, and Stefano Dall’Acqua. 2019. "Triterpene Acid and Phenolics from Ancient Apples of Friuli Venezia Giulia as Nutraceutical Ingredients: LC-MS Study and In Vitro Activities" Molecules 24, no. 6: 1109. https://doi.org/10.3390/molecules24061109
APA StyleSut, S., Zengin, G., Maggi, F., Malagoli, M., & Dall’Acqua, S. (2019). Triterpene Acid and Phenolics from Ancient Apples of Friuli Venezia Giulia as Nutraceutical Ingredients: LC-MS Study and In Vitro Activities. Molecules, 24(6), 1109. https://doi.org/10.3390/molecules24061109