Effect of the Incorporation of Functionalized Cyclodextrins in the Liposomal Bilayer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Liposome Size
2.2. Viscosity of the Membrane
2.3. Liposome Stability
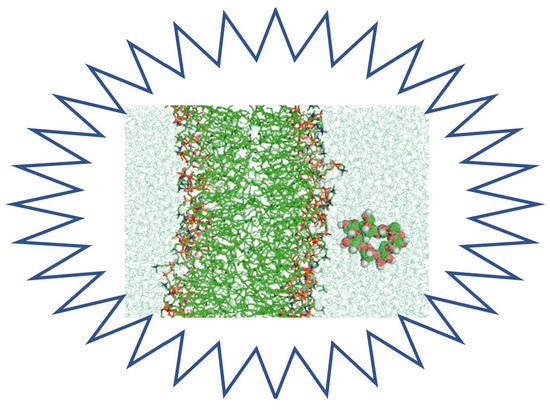
2.4. Molecular Dynamics Simulation
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Instruments
4.3. POPC Liposome Preparation
4.4. Fluorimetric Measurements
4.5. Molecular Dynamics Simulations
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- McCormack, B.; Gregoriadis, G. Drugs-in-cyclodextrins-in liposomes: A novel concept in drug delivery. Int. J. Pharm. 1994, 112, 249–258. [Google Scholar] [CrossRef]
- Aloisio, C.; Antimisiaris, S.G.; Longhi, M.R. Liposomes containing cyclodextrins or meglumine to solubilize and improve the bioavailability of poorly soluble drugs. J. Mol. Liq. 2017, 229, 106–113. [Google Scholar] [CrossRef]
- Gharib, R.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Drug-in-cyclodextrin-in-liposomes as a carrier system for volatile essential oil components: Application to anethole. Food Chem. 2017, 218, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Lounès-Hadj Sahraoui, A.; Bourdon, N.; Laruelle, F.; Fontaine, J.; Auezova, L.; Fourmentin, S. Solubility, photostability and antifungal activity of phenylpropanoids encapsulated in cyclodextrins. Food Chem. 2016, 196, 518–525. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Azzi, J.; Jraij, A.; Auezova, L.; Fourmentin, S.; Greige-Gerges, H. Novel findings for quercetin encapsulation and preservation with cyclodextrins, liposomes, and drug-in-cyclodextrin-in-liposomes. Food Hydrocolloids 2018, 81, 328–340. [Google Scholar] [CrossRef]
- Gharib, R.; Greige-Gergesa, H.; Fourmentin, S.; Charcosset, C. Hydroxypropyl-β-cyclodextrin as a membrane protectant during freeze drying of hydrogenated and non-hydrogenated liposomes and molecule-in-cyclodextrin-in- liposomes: Application to trans-anethole. Food Chem. 2018, 267, 67–74. [Google Scholar] [CrossRef]
- Azzi, J.; Auezova, L.; Danjou, P.-E.; Fourmentin, S.; Greige-Gergesa, H. First evaluation of drug-in-cyclodextrin-in-liposomes as an encapsulating system for nerolidol. Food Chem. 2018, 255, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Matloob, A.H.; Mourtas, S.; Klepetsanis, P.; Antimisiaris, S.G. Increasing the stability of curcumin in serum with liposomes or hybrid drug-in-cyclodextrin-in-liposome systems: A comparative study. Int. J. Pharm. 2014, 476, 108–115. [Google Scholar] [CrossRef]
- Bhatt, P.; Lalani, R.; Vhora, I.; Patil, S.; Amrutiya, J.; Misra, A.; Mashru, R. Liposomes encapsulating native and cyclodextrin enclosed paclitaxel: Enhanced loading efficiency and its pharmacokinetic evaluation. Int. J. Pharm. 2018, 536, 95–107. [Google Scholar] [CrossRef]
- Yakavets, I.; Lassalle, H.-P.; Scheglmann, D.; Wiehe, A.; Zorin, V.; Bezdetnaya, L. Temoporfin-in-cyclodextrin-in-liposome. A new approach for anticancer drug delivery: The optimization of composition. Nanomaterials 2018, 8, 847. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- de Miranda, J.C.; Martins, T.E.A.; Veiga, F.; Ferraz, H.G. Cyclodextrins and ternary complexes: Technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef]
- Liu, Y.; Han, B.H.; Li, B.; Zhang, Y.M.; Zhao, P.; Chen, Y.T.; Wada, T.; Inoue, Y. Molecular recognition study on supramolecular systems. Synthesis of modified cyclodextrin and their inclusion complexation thermodynamics with L-tryptophan and some naphthalene derivatives. J. Org. Chem. 1998, 63, 1444–1454. [Google Scholar] [CrossRef]
- Qu, Q.; Tucker, E.; Christian, S.D. Sulfoalkyl ether β-cyclodextrin derivatives: Synthesis and characterizations. J. Incl. Phenom. Macrocycl. Chem. 2002, 43, 213–222. [Google Scholar] [CrossRef]
- Grachev, M.K.; Kurochkina, G.I.; Mishina, V.Y.; Mustafin, I.G.; Nifant’ev, E.E. Phosphorylated derivatives on the basis of per-6-O-(tert-butyldimethylsilyl)cyclodextrins. Russ. J. Gen. Chem. 1999, 69, 1702–1707. [Google Scholar]
- Brandt, L.; Felcht, U.H. Water Soluble Mixed Ether of Beta-cyclodextrin, and Process for Its Preparation. Patent EP0146841(A2), 3 July 1985. [Google Scholar]
- Chen, J.; Lu, W.L.; Gu, W.; Lu, S.S.; Chen, Z.-P.; Cai, B.C.; Yang, X.-X. Drug-in-cyclodextrin-in-liposomes: A promising delivery system for hydrophobic drugs. Expert Opin. Drug Deliv. 2014, 11, 565–577. [Google Scholar] [CrossRef]
- Gharib, R.; Greige-Gerges, H.; Fourmentin, S.; Charcosset, C.; Auezova, L. Liposomes incorporating cyclodextrin-drug inclusion complexes: Current state of knowledge. Carbohydr. Polym. 2015, 129, 175–186. [Google Scholar] [CrossRef]
- Palazzo, C.; Laloy, J.; Delvigne, A.-S.; Nys, G.; Fillet, M.; Dogne, J.-M.; Pequeux, C.; Foidart, J.-M.; Evrard, B.; Piel, G. Development of injectable liposomes and drug-in-cyclodextrin-in-liposome formulations encapsulating estetrol to prevent cerebral ischemia of premature babies. Eur. J. Pharm. Sci. 2019, 127, 52–59. [Google Scholar] [CrossRef]
- Bragagni, M.; Maestrelli, F.; Mennini, N.; Ghelardini, C.; Mura, P. Liposomal formulations of prilocaine: Effect of complexation with hydroxypropyl-β-cyclodextrin on drug anesthetic efficacy. J. Liposome Res. 2010, 20, 315–322. [Google Scholar] [CrossRef]
- Sebaaly, C.; Charcosset, C.; Stainmesse, S.; Fessi, H.; Greige-Gerges, H. Clove essential oil-in-cyclodextrin-in-liposomes in the aqueous and lyophilized states: From laboratory to large scale using a membrane contactor. Carbohydr. Polym. 2016, 138, 75–85. [Google Scholar] [CrossRef]
- Gasbarri, C.; Guernelli, S.; Boncompagni, S.; Angelini, G.; Siani, G.; De Maria, P.; Fontana, A. Fine-tuning of POPC liposomal leakage by the use of β-cyclodextrin and several hydrophobic guests. J. Liposome Res. 2010, 20, 202–210. [Google Scholar] [CrossRef]
- Hatzi, P.; Mourtas, S.; Klepetsanis, P.G.; Antimisiaris, S.G. Integrity of liposomes in presence of cyclodextrins: Effect of liposome type and lipid composition. Int. J. Pharm. 2007, 333, 167–176. [Google Scholar] [CrossRef]
- Maestrelli, F.; González-Rodríguez, M.L.; Rabasco, A.M.; Ghelardini, C.; Mura, P. New “drug-in cyclodextrin-in deformable liposomes” formulations to improve the therapeutic efficacy of local anaesthetics. Int. J. Pharm. 2010, 395, 222–231. [Google Scholar] [CrossRef]
- Angelini, G.; Campestre, C.; Boncompagni, S.; Gasbarri, C. Liposomes entrapping β-cyclodextrin/ibuprofen inclusion complex: Role of the host and the guest on the bilayer integrity and microviscosity. Chem. Phys. Lipids 2017, 209, 61–65. [Google Scholar] [CrossRef]
- Ghariba, R.; Fourmentin, S.; Charcosset, C.; Greige-Gergesa, H. Effect of hydroxypropyl-β-cyclodextrin on lipid membrane fluidity, stability and freeze-drying of liposomes. J. Drug Deliv. Sci. Technol. 2018, 44, 101–107. [Google Scholar] [CrossRef]
- Wenz, G. Influence of intramolecular hydrogen bonds on the binding potential of methylated β-cyclodextrin derivatives. Belstein J. Org. Chem. 2012, 8, 1890–1895. [Google Scholar] [CrossRef]
- Szejtli, J.; Lipták, A.; Jodál, I.; Fügedi, P.; Nánási, P.; Neszmélyi, A. Synthesis and 13C-NMR spectroscopy of methylated beta-cyclodextrins. Starch Staerke 1980, 32, 165–169. [Google Scholar] [CrossRef]
- Nishijo, J.; Mizuno, H. Interactions of cyclodextrins with DPPC liposomes. Differential scanning calorimetry study. Chem. Pharm. Bull. 1998, 46, 120–124. [Google Scholar] [CrossRef]
- Wang, X.; Fan, H.; Zhang, F.; Qi, Y.; Qiu, W.; Yang, F.; Tang, J.; He, P. Synthesis of a β-cyclodextrin derivate and its molecular recognition behavior on modified glassy carbon electrode by diazotization. Tetrahedron 2010, 66, 7815–7820. [Google Scholar] [CrossRef]
- Takeo, K.; Mitoh, H.; Uemura, K. Selective chemical modification of cyclomaltooligosaccha-rides via tert-butyldimethylsilylation. Carbohydr. Res. 1989, 187, 203–221. [Google Scholar] [CrossRef]
- Puglisi, G.; Fresta, M.; Ventura, C.A. Interaction of natural and modified β-cyclodextrins with a biological membrane model of dipalmitoylphosphatidylcholine. J. Colloid Interface Sci. 1996, 180, 542–547. [Google Scholar] [CrossRef]
- Besseau, F. Hydrogen-bond basicity of esters, lactones and carbonates. J. Chem. Soc. Perkin Trans. 1994, 2, 485–489. [Google Scholar] [CrossRef]
- De Maria, P.; Fontana, A.; Gasbarri, C.; Velluto, D. Effects of fullerene guests on the stability of 1-palmitoyl-2-oleoylphosphatidylcholine liposomes. Soft Matter 2006, 2, 595–602. [Google Scholar] [CrossRef]
- Silvander, M.; Bergstrand, N.; Edwards, K. Linkage identity is a major factor in determining the effect of PEG-ylated surfactants on permeability of phosphatidylcholine liposomes. Chem. Phys. Lipids 2003, 126, 77–83. [Google Scholar] [CrossRef]
- Bahri, M.A.; Heyne, B.L.; Hans, P.; Mouithys Mickalad, A.A.; Hoebeke, M.D. Quantification of lipid bilayer effective microviscosity and fluidity effect induced by propofol. Biophys. Chem. 2005, 114, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Pownall, H.J.; Smith, L.C. Viscosity of the hydrocarbon region of micelles. Measurement by excimer fluorescence. J. Am. Chem. Soc. 1973, 95, 3136–3140. [Google Scholar] [CrossRef]
- Birks, J.B. Excimers. Rep. Prog. Phys. 1975, 38, 903–974. [Google Scholar] [CrossRef]
- Turro, N.J.; Lei, X.-G.; Ananthapadmanabhan, K.P.; Aronson, M. Spectroscopic probe analysis of protein-surfactant interactions: The BSA/SDS system. Langmuir 1995, 11, 2525–2533. [Google Scholar] [CrossRef]
- Aschi, M.; D’Archivio, A.A.; Fontana, A.; Formiglio, A. Physicochemical properties of fluorescent probes: Experimental and computational determination of the overlapping pKa values of carboxyfluorescein. J. Org. Chem. 2008, 73, 3411–3417. [Google Scholar] [CrossRef]
- Kashchiev, D.; Exerova, D. Bilayer lipid membrane permeation and rupture due to hole formation. Biochim. Biophys. Acta Biomembr. 1983, 732, 133–145. [Google Scholar] [CrossRef]
- Iwata, T.; Lee, S.; Oishi, O.; Aoyagi, H.; Ohno, M.; Anzai, K.; Kirino, Y.; Sugihara, G. Design and synthesis of amphipathic 3(10)-helical peptides and their interactions with phospholipid bilayers and ion channel formation. J. Biol. Chem. 1994, 269, 4928–4933. [Google Scholar]
- Scrimin, P.; Tecilla, P.; Tonellato, U.; Veronese, A.; Crisma, M.; Formaggio, F.; Toniolo, C. Zinc(II) as an allosteric regulator of liposomal membrane permeability induced by synthetic template-assembled tripodal polypeptides. Chem. Eur. J. 2002, 8, 2753–2763. [Google Scholar] [CrossRef]
- De Maria, P.; Filippone, P.; Fontana, A.; Gasbarri, C.; Siani, G.; Velluto, D. Cardanol as a replacement for cholesterol into the lipid bilayer of POPC liposomes. Colloid Surf. B Biointerfaces 2005, 40, 11–18. [Google Scholar] [CrossRef]
- Angelini, G.; Boncompagni, S.; De Maria, P.; Fontana, A.; Gasbarri, C.; Siani, G. Kinetic evaluation of the effect of layer by layer deposition of polyeletrolytes on the stability of POPC liposomes. Colloid Surf. A Physicochem. Eng. Asp. 2008, 322, 234–238. [Google Scholar] [CrossRef]
- Zappacosta, R.; Semeraro, M.; Baroncini, M.; Silvi, S.; Aschi, M.; Credi, A.; Fontana, A. Liposome destabilization by a 2,7-diazapyrenium derivative through formation of transient pores in the lipid bilayer. Small 2010, 6, 952–959. [Google Scholar] [CrossRef]
- Velluto, D.; Gasbarri, C.; Angelini, G.; Fontana, A. Use of simple kinetic and reaction order measurements for the evaluation of the mechanism of surfactant-liposome interactions. J. Phys. Chem. B 2011, 115, 8130–8137. [Google Scholar] [CrossRef]
- Khuntawee, W.; Wolschann, P.; Rungrotmongkol, T.; Wong-Ekkabut, J.; Hannongbua, S. Molecular dynamics simulations of the interaction of beta-cyclodextrin with a lipid bilayer. J. Chem. Inf. Model. 2015, 55, 1894–1902. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, J.-S.; Lee, Y.-S.; Kim, M. Effect of drug substances on the microviscosity of lipid bilayer of liposomal membrane. Arch. Pharm. Res. 1990, 13, 192–197. [Google Scholar] [CrossRef]
- Lopez, C.A.; de Vries, A.H.; Marrink, S.J. Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes. Sci. Rep. 2013, 3, 2071. [Google Scholar] [CrossRef]
- Ivanov, P.M.; Jaime, C. Insights into the structure of large-ring cyclodextrins through molecular dynamics simulations in solution. J. Phys. Chem. B 2004, 108, 6261–6274. [Google Scholar] [CrossRef]
- Nutho, B.; Khuntawee, W.; Rungnim, C.; Pongsawasdi, P.; Wolschann, P.; Karpfen, A.; Kungwan, N.; Rungrotmongkol, T. Binding mode and free energy prediction of fisetin/β-cyclodextrin inclusion complexes. Beilstein J. Org. Chem. 2014, 10, 2789–2799. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tan, T.; Feng, W.; van der Spoel, D. Molecular recognition in different environments: β-cyclodextrin dimer formation in organic solvents. J. Phys. Chem. B 2012, 116, 12684–12693. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, W.; Li, C.; Tan, T. Investigation of the inclusions of puerarin and daidzin with β-cyclodextrin by molecular dynamics simulation. J. Phys. Chem. B 2010, 114, 4876–4883. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Van der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Schaftenaar, G.; Noordik, J.H. Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput. Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef]
- Van Gunsteren, W.F.; Billeter, S.R.; Eising, A.A.; Hunenberger, P.H.; Kruger, P.; Mark, A.E.; Scott, W.R.P.; Tironi, I.G. Biomolecular Simulation: The GROMOS96 Manual and User Guide; Hochschulverlag AG an der ETH Zurich: Zurich, Switzerland, 1996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2014, 393, 51–57. [Google Scholar] [CrossRef]
- Kukol, A. Lipid models for united-atom molecular dynamics simulations of proteins. J. Chem. Theory Comput. 2009, 5, 615–626. [Google Scholar] [CrossRef]
- Berendsen, J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Interaction models for water in relation to protein hydration. In Intermolecular Forces; Pullman, B., Ed.; Reidel: Dordrecht, The Netherlands, 1981; pp. 331–342. [Google Scholar]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [Green Version]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Frajie, J.C.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef] [Green Version]
- Darden, T.A.; York, D.M.; Pedersen, L.G. Particle mesh Ewald: An N·log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Roux, B. The calculation of the potential of mean force using computer simulations. Comput. Phys. Commun. 1995, 91, 275–282. [Google Scholar] [CrossRef]
- Kumar, S.; Rosenberg, J.M.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A. The weighted histogram analysis method for free energy calculations in biomolecules. I. The method. J. Comput. Chem. 1992, 13, 1011–1021. [Google Scholar] [CrossRef]
- Hub, J.S.; de Groot, B.L.; van der Spoel, D. g_wham: A free weighted histogram analysis implementation including robust error and autocorrelation estimates. J. Chem. Theory Comput. 2010, 6, 3713–3720. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds TMCD and DACD are available from the authors. |




| Entry | Liposome Composition | POPC/CD [a] | Diameter (nm) | Polydispersity |
|---|---|---|---|---|
| 1 | POPC | 118.0 ± 0.5 | 0.02 ± 0.01 | |
| 2 | POPC/β-CD | 12 | 131.6 ± 1.5 | 0.05 ± 0.01 |
| 3 | POPC/β-CD | 5 | 130.5 ± 2.3 | 0.05 ± 0.02 |
| 4 | POPC/β-CD | 2.5 | 132.2 ± 0.7 | 0.08 ± 0.01 |
| 5 | POPC/TMCD | 12 | 128.6 ± 4.7 | 0.18 ± 0.01 |
| 6 | POPC/TMCD | 5 | 136.6 ± 1.0 | 0.13 ± 0.01 |
| 7 | POPC/TMCD | 2.5 | 144.1 ± 3.0 | 0.11 ± 0.01 |
| 8 | POPC/DACD | 12 | 144.6 ± 2.3 | 0.11 ± 0.02 |
| 9 | POPC/DACD | 5 | 160.7 ± 4.2 | 0.19 ± 0.03 |
| 10 | POPC/DACD | 2.5 | 185.8 ± 0.6 | 0.29 ± 0.01 |
| Entry | Liposome Composition | POPC/CD [b] | C′h × 105 (M) at 25.0 ± 0.1 °C | C′h × 105 (M) at 37.0 ± 0.1 °C |
|---|---|---|---|---|
| 1 | POPC | 13.80 ± 0.10 | 4.52 ± 0.01 | |
| 2 | POPC/β-CD | 12 | 11.70 ± 0.40 | 8.31 ± 0.58 |
| 3 | POPC/β-CD | 5 | 7.44 ± 0.11 | 5.37 ± 0.15 |
| 4 | POPC/β-CD | 2.5 | 7.16 ± 0.08 | 6.14 ± 0.15 |
| 5 | POPC/TMCD | 12 | 14.40 ± 0.40 | 11.70 ± 0.10 |
| 6 | POPC/TMCD | 5 | 13.90 ± 0.05 | 9.37 ± 0.20 |
| 7 | POPC/TMCD | 2.5 | 6.75 ± 0.54 | 5.20 ± 0.18 |
| 8 | POPC/DACD | 12 | 15.10 ± 0.60 | 11.00 ± 0.90 |
| 9 | POPC/DACD | 5 | 15.80 ± 1.50 | 11.00 ± 0.09 |
| 10 | POPC/DACD | 2.5 | 8.07 ± 0.20 | 5.76 ± 0.11 |
| Entry | Liposome Composition | POPC/CD [a] | 10−4 kobs (s−1) at 25.0 ± 0.1 °C | 10−4 kobs (s−1) at 37.0 ± 0.1 °C |
|---|---|---|---|---|
| 1 | POPC | 1.31 ± 0.05 | 0.90 ± 0.04 | |
| 2 | POPC/β-CD | 12 | 1.18 ± 0.05 | 1.15 ± 0.05 |
| 3 | POPC/β-CD | 5 | 1.18 ± 0.13 | 1.40 ± 0.19 |
| 4 | POPC/β-CD | 2.5 | 0.28 ± 0.03 | 0.93 ± 0.02 |
| 5 | POPC/TMCD | 12 | 1.48 ± 0.05 | 1.24 ± 0.06 |
| 6 | POPC/TMCD | 5 | 0.89 ± 0.03 | 0.60 ± 0.03 |
| 7 | POPC/TMCD | 2.5 | 0.32 ± 0.07 | 0.33 ± 0.08 |
| 8 | POPC/DACD | 12 | 1.40 ± 0.15 | 1.54 ± 0.23 |
| 9 | POPC/DACD | 5 | 1.62 ± 0.15 | 1.74 ± 0.16 |
| 10 | POPC/DACD | 2.5 | 0.30 ± 0.05 | 0.58 ± 0.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappacosta, R.; Cornelio, B.; Pilato, S.; Siani, G.; Estour, F.; Aschi, M.; Fontana, A. Effect of the Incorporation of Functionalized Cyclodextrins in the Liposomal Bilayer. Molecules 2019, 24, 1387. https://doi.org/10.3390/molecules24071387
Zappacosta R, Cornelio B, Pilato S, Siani G, Estour F, Aschi M, Fontana A. Effect of the Incorporation of Functionalized Cyclodextrins in the Liposomal Bilayer. Molecules. 2019; 24(7):1387. https://doi.org/10.3390/molecules24071387
Chicago/Turabian StyleZappacosta, Romina, Benedetta Cornelio, Serena Pilato, Gabriella Siani, François Estour, Massimiliano Aschi, and Antonella Fontana. 2019. "Effect of the Incorporation of Functionalized Cyclodextrins in the Liposomal Bilayer" Molecules 24, no. 7: 1387. https://doi.org/10.3390/molecules24071387
APA StyleZappacosta, R., Cornelio, B., Pilato, S., Siani, G., Estour, F., Aschi, M., & Fontana, A. (2019). Effect of the Incorporation of Functionalized Cyclodextrins in the Liposomal Bilayer. Molecules, 24(7), 1387. https://doi.org/10.3390/molecules24071387