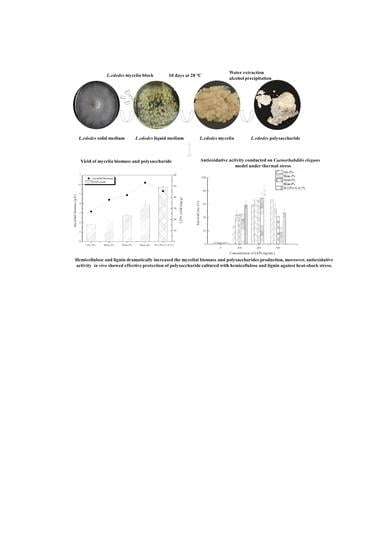
The Effect of Hemicellulose and Lignin on Properties of Polysaccharides in Lentinus edodes and Their Antioxidant Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of Hemicellulose and Lignin on Mycelial Biomass and LEPs Yield
2.2. Chemical Analysis of LEPs
2.3. Analysis of Monosaccharide Composition
2.4. Molecular Weight Analysis
2.5. UV-Visible and FTIR Spectra
2.6. Antioxidant Activity Assays
2.6.1. Antioxidant Activity In Vitro
2.6.2. Antioxidant Activity In Vivo
3. Materials and Methods
3.1. Materials and Reagents
3.2. Culture and Experimental Design
3.3. Mycelia Preparation and Polysaccharide Extraction
3.4. Characterization of L. edodes Polysaccharides
3.4.1. Determination of Total Carbohydrate, Protein, Uronic Acid and Total Polyphenols Content
3.4.2. Determination of Monosaccharide Composition
3.4.3. Determination of Molecular Weight
3.4.4. UV-Visible Spectra and Fourier-Transform Infrared Spectra
3.5. Evaluation of Antioxidant Activity In Vitro
3.5.1. Assay of DPPH Radical Scavenging Activity
3.5.2. Assay of Hydroxyl Radical Scavenging Activity
3.5.3. Assay of ABTS Radical Scavenging Activity
3.6. Evaluation of Antioxidant Activity In Vivo
3.6.1. Caenorhabditis Elegans Strains, Culture and Synchronization
3.6.2. Heat Shock Stress Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P.C.K. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocolloid. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B. Lentinus edodes: A macrofungus with pharmacological activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Chen, Z.; Gao, X.; Chen, Y.; Xue, Z.; Guo, Q.; Ma, Q.; Chen, H. Physicochemical properties of polysaccharides from Lentinus edodes under high pressure cooking treatment and its enhanced anticancer effects. Int. J. Biol. Macromol. 2018, 115, 994–1001. [Google Scholar] [CrossRef]
- Ya, G. A Lentinus edodes polysaccharide induces mitochondrial-mediated apoptosis in human cervical carcinoma HeLa cells. Int. J. Biol. Macromol. 2017, 103, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, H.; Tang, J.; Chen, J.; Zhang, X. Polysaccharides in Lentinus edodes: Isolation, structure, immunomodulating activity and future prospective. Crit Rev. Food Sci Nutr. 2014, 54, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, Z.; Gan, D.; Fan, J.; Dai, Z.; Wang, X.; Hu, B.; Ye, H.; Abid, M.; Zeng, X. Influences of carbon sources on the biomass, production and compositions of exopolysaccharides from Paecilomyces hepiali HN1. Biomass Bioenergy 2014, 67, 260–269. [Google Scholar] [CrossRef]
- Duan, X.; Chi, Z.; Wang, L.; Wang, X. Influence of different sugars on pullulan production and activities of α-phosphoglucose mutase, UDPG-pyrophosphorylase and glucosyltransferase involved in pullulan synthesis in Aureobasidium pullulans Y68. Carbohyd. Polym. 2008, 73, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, X.; Tang, Y.; Dong, F.; Sun, H.; Chen, L.; Zhang, Y. Effects of cultural medium on the formation and antitumor activity of polysaccharides by Cordyceps gunnii. J. Biosci. Bioeng. 2016, 122, 494–498. [Google Scholar] [CrossRef]
- Chen, L.; Gong, Y.; Cai, Y.; Liu, W.; Zhou, Y.; Xiao, Y.; Xu, Z.; Liu, Y.; Lei, X.; Wang, G.; et al. Genome sequence of the edible cultivated mushroom Lentinula edodes (Shiitake) reveals insights into lignocellulose degradation. PLoS ONE 2016, 11, e0160336. [Google Scholar] [CrossRef]
- Pire, D.G.; Wright, J.E.; Albertó, E. Cultivation of shiitake using sawdust from widely available local woods in Argentina. Micol. Apl. Int. 2001, 13, 87–91. [Google Scholar]
- Philippoussis, A.; Diamantopoulou, P.; Papadopoulou, K.; Lakhtar, H.; Roussos, S.; Parissopoulos, G.; Papanikolaou, S. Biomass, laccase and endoglucanase production by Lentinula edodes during solid state fermentation of reed grass, bean stalks and wheat straw residues. World J. Microbiol. Biotechnol. 2011, 27, 285–297. [Google Scholar] [CrossRef]
- Gaitan-Hernandez, R.; Esqueda, M.; Gutierrez, A.; Beltran-Garcia, M. Quantitative changes in the biochemical composition of lignocellulosic residues during the vegetative growth of Lentinula edodes. Braz. J. Microbiol. 2011, 42, 30–40. [Google Scholar] [CrossRef]
- Thetsrimuang, C.; Khammuang, S.; Chiablaem, K.; Srisomsap, C.; Sarnthima, R. Antioxidant properties and cytotoxicity of crude polysaccharides from Lentinus polychrous Lév. Food Chem. 2011, 128, 634–639. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, L.; Qian, Y.; Yan, L. Effect of Lentinus edodes polysaccharide on oxidative stress, immunity activity and oral ulceration of rats stimulated by phenol. Carbohyd. Polym. 2009, 75, 115–118. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Shen, M.; Nie, S.; Gong, B.; Li, H.; Zhao, Q.; Li, W.; Xie, M. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocolloid. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Kawamura, N.; Goto, M.; Nakamura, Y. Effect of lignin and its precursors on vegetative growth and fruiting body formation in Lentinus edodes. Nippon Kingakukai Kaiho 1983, 24, 213–222. [Google Scholar]
- Medany, G.M. Optimization of submerged culture conditions for mycelial biomass production by shiitake mushroom (Lentinus edodes). Res. J. Agric. Biol. Sci. 2011, 7, 350–356. [Google Scholar]
- Cai, Y. Expression profiling analysis of lignocellulose degrading enzymes in Lentinula edodes grown on different carbon sources. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2017. [Google Scholar]
- Lee, B.C.; Bae, J.T.; Pyo, H.B.; Choe, T.B.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible Basidiomycete Grifola frondosa. Enzym. Microb. Tech. 2004, 35, 369–376. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, W.C.; Park, S.J.; Lee, K.E.; Shin, W.C.; Hong, E.K. Culture conditions and medium components for the production of mycelial biomass and exo-polysaccharides with Paecilomyces japonica in liquid culture. J. Biosci. Bioeng. 2013, 115, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.C. Oxidative coupling of tyrosine and ferulic acid residues: Intra- and extra-protoplasmic occurrence, predominance of trimers and larger products, and possible role in inter-polymeric cross-linking. Phytochem. Rev. 2004, 3, 97–111. [Google Scholar] [CrossRef]
- Xu, C.; Yu, J.; Zhao, S.; Wu, S.; He, P.; Jia, X.; Liu, Y.; Mao, D. Effect of carbon source on production, characterization and bioactivity of exopolysaccharide produced by Phellinus vaninii Ljup. An. Da Acad. Bras. De Ciências 2017, 89, 2033–2043. [Google Scholar] [CrossRef]
- Lee, M.; Chao, C.; Lu, M. Effect of carbohydrate-based media on the biomass, polysaccharides molecular weight distribution and sugar composition from Pycnoporus sanguineus. Biomass Bioenerg. 2012, 47, 37–43. [Google Scholar] [CrossRef]
- Xie, J.; Xie, M.; Nie, S.; Shen, M.; Wang, Y.; Li, C. Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Wang, A.; Yu, Z.; Xu, Y.; Yang, Y. Purification, characterization and bioactivity determination of a novel polysaccharide from pumpkin (Cucurbita moschata) seeds. Food Hydrocolloid. 2017, 66, 357–364. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, D.; Zhang, H.; Li, Q.; Zhang, Z.; Liu, Y.; Zhou, S.; Wang, W.; Li, Z.; Yang, Y. Effects of Atmospheric and Room Temperature Plasma (ARTP) Mutagenesis on Physicochemical Characteristics and Immune Activity In Vitro of Hericium erinaceus Polysaccharides. Molecules 2019, 24, 262. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohyd. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Lo, T.C.; Chang, C.A.; Chiu, K.; Tsay, P.; Jen, J. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohyd. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Xu, X.; Hu, Y.; Quan, L. Production of bioactive polysaccharides by Inonotus obliquus under submerged fermentation supplemented with lignocellulosic biomass and their antioxidant activity. Bioproc. Biosyst. Eng. 2014, 37, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ding, S.; Fan, L. Antioxidant activities of five polysaccharides from Inonotus obliquus. Int. J. Biol. Macromol. 2012, 50, 1183–1187. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, M.; Chen, Y.; Lou, Y.; Luo, R.; Chen, J.; Zhang, Y.; Li, J.; Wang, W. Optimization of the polysaccharide hydrolysate from Auricularia auricula with antioxidant activity by response surface methodology. Int. J. Biol. Macromol. 2018, 113, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, R.; Li, H.; Xiang, Y.; Xiao, L.; Hu, M.; Ma, F.; Ma, C.W.; Huang, Z. Antioxidant and neuroprotective effects of Dictyophora indusiata polysaccharide in Caenorhabditis elegans. J. Ethnopharmacol. 2016, 192, 413–422. [Google Scholar] [CrossRef]
- Voisine, C.; Varma, H.; Walker, N.; Bates, E.A.; Stockwell, B.R.; Hart, A.C. Identification of potential therapeutic drugs for huntington’s disease using Caenorhabditis elegans. Plos ONE 2007, 6, e504. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cheng, H.; Xu, Z.; Shen, S.; Yuan, M.; Liu, J.; Ding, C. Thermal stress resistance and aging effects of Panax notoginseng polysaccharides on Caenorhabditis elegans. Int. J. Biol. Macromol. 2015, 81, 188–194. [Google Scholar] [CrossRef]
- Cao, X.; Liu, R.; Liu, J.; Huo, Y.; Yang, W.; Zeng, M.; Yang, C. A novel polysaccharide from Lentinus edodes mycelia exhibits potential antitumor activity on laryngeal squamous cancer cell line Hep-2. Appl. Biochem. Biotech. 2013, 171, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Ye, H.; Hu, B.; Zhou, L.; Jabbar, S.; Zeng, X.; Shen, W. Optimization of extraction, characterization and antioxidant activity of polysaccharides from Brassica rapa L. Int. J. Biol. Macromol. 2016, 82, 979–988. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, S.; Wang, R.; Li, D.; Hu, Y.; Nie, J.; Zhao, X.; Wang, Q.; Chen, Y.; Zheng, Y.; et al. Polysaccharides from Rhizoma Panacis Majoris and its anti-oxidant activity. Int. J. Biol. Macromol. 2016, 86, 756–763. [Google Scholar] [CrossRef]
- Branner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 1, 71–94. [Google Scholar]
- Kim, D.K.; Jeon, H.; Cha, D.S. 4-Hydroxybenzoic acid-mediated lifespan extension in Caenorhabditis elegans. J. Funct. Foods 2014, 7, 630–640. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |







| Samples | Mycelium Colony Diameter (mm) | Average Growth Rate (mm/day) | |||
|---|---|---|---|---|---|
| Day 1 | Day 4 | Day 8 | Day 12 | ||
| Hem-2.0% | 10 ± 0.03 | 24 ± 0.03 | 47 ± 0.12 | 59 ± 0.06 | 4.08 |
| H-2.0% + L-0.05% | 10 ± 0.06 | 19 ± 0.11 | 47 ± 0.16 | 62 ± 0.1 | 4.33 |
| H-2.0% + L-0.1% | 10 ± 0.06 | 25 ± 0.55 | 60 ± 0.54 | 87 ± 0.21 | 6.41 |
| H-2.0% + L-0.2% | 10 ± 0.01 | 15 ± 0.11 | 39 ± 0.15 | 48 ± 0.17 | 3.16 |
| Samples | Protein (%) | Uronic Acid (%) | Total Phenolics (mg GAE/g) 2 |
|---|---|---|---|
| Glc-2% | 0.64 ± 0.18 | 6.77 ± 0.27 | 2.44 ± 0.33 |
| Hem-2% | 1.03 ± 0.13 | 6.88 ± 0.31 | 7.39 ± 0.21 |
| Hem-3% | 1.42 ± 0.05 | 7.49 ± 0.08 | 7.48 ± 0.26 |
| Hem-4% | 1.80 ± 0.08 | 7.54 ± 0.22 | 10.90 ± 0.91 |
| H-2.0% + L-0.1% | 2.64 ± 0.17 | 5.23±0.72 | 11.91 ± 0.69 |
| Samples | Ara (%) | Glc (%) | Xyl (%) | Gal (%) | Man (%) |
|---|---|---|---|---|---|
| Glc-2% | 19.49 | 15.79 | 15.79 | 39.18 | 9.75 |
| Hem-2% | 21.14 | 15.64 | 25.16 | 32.77 | 5.29 |
| Hem-3% | 23.47 | 11.74 | 30.28 | 29.58 | 4.93 |
| Hem-4% | 23.04 | 13.59 | 35.94 | 23.27 | 4.15 |
| Hem-2.0% + L-0.1% | 18.69 | 26.36 | 17.01 | 31.78 | 6.17 |
| Samples | Peak1 | Peak2 | Peak3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mw | Mn | Mw/Mn | Ratio (%) | Mw | Mn | Mw/Mn | Ratio (%) | Mw | Mn | Mw/Mn | Ratio (%) | |
| Glc-2% | 35.43 | 30.54 | 1.16 | 64.20 | 12.05 | 10.82 | 1.11 | 17.06 | 1.31 | 1.16 | 1.117 | 18.74 |
| Hem-2% | 16.36 | 13.3 | 1.23 | 67.83 | - | - | - | - | 1.58 | 1.23 | 1.28 | 32.17 |
| Hem-3% | 21.47 | 15.9 | 1.35 | 77.34 | - | - | - | - | 1.00 | 0.94 | 1.06 | 22.66 |
| Hem-4% | 19.63 | 16.36 | 1.20 | 63.18 | - | - | - | - | 1.33 | 1.17 | 1.14 | 36.82 |
| H-2.0% + L-0.1% | 20.71 | 17.7 | 1.17 | 40.36 | 8.42 | 7.32 | 1.15 | 34.89 | 0.92 | 0.63 | 1.46 | 24.75 |
| Carbon source | Concentration (m/v) | Abbreviation | pH |
|---|---|---|---|
| hemicellulose | 2%, 3%, 4% | Hem-2%, 3%, 4% | 6 |
| hemicellulose + lignin | 2.0% + 0.05% | H-2.0% + L-0.05% | |
| 2.0% + 0.1% | H-2.0% + L-0.1% | ||
| 2.0% + 0.2% | H-2.0% + L-0.2% | ||
| glucose | 2% | Glc-2% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Jia, X.; Yin, L.; Cheng, Y.; Miao, Y.; Zhang, X. The Effect of Hemicellulose and Lignin on Properties of Polysaccharides in Lentinus edodes and Their Antioxidant Evaluation. Molecules 2019, 24, 1834. https://doi.org/10.3390/molecules24091834
Wu F, Jia X, Yin L, Cheng Y, Miao Y, Zhang X. The Effect of Hemicellulose and Lignin on Properties of Polysaccharides in Lentinus edodes and Their Antioxidant Evaluation. Molecules. 2019; 24(9):1834. https://doi.org/10.3390/molecules24091834
Chicago/Turabian StyleWu, Feifei, Xin Jia, Lijun Yin, Yongqiang Cheng, Yuxin Miao, and Xiuqing Zhang. 2019. "The Effect of Hemicellulose and Lignin on Properties of Polysaccharides in Lentinus edodes and Their Antioxidant Evaluation" Molecules 24, no. 9: 1834. https://doi.org/10.3390/molecules24091834
APA StyleWu, F., Jia, X., Yin, L., Cheng, Y., Miao, Y., & Zhang, X. (2019). The Effect of Hemicellulose and Lignin on Properties of Polysaccharides in Lentinus edodes and Their Antioxidant Evaluation. Molecules, 24(9), 1834. https://doi.org/10.3390/molecules24091834