Antiviral Effects of Houttuynia cordata Polysaccharide Extract on Murine Norovirus-1 (MNV-1)—A Human Norovirus Surrogate
Abstract
:1. Introduction
2. Results
2.1. Chemical Analyses of HP, HWE, and HEE
2.2. Cytotoxicity Assay
2.3. Anti-Viral Activity of HP, HWE, and HEE
2.4. Time-Dependence Experiment of HP, HWE, and HEE on Viral Count Reduction
2.5. Viral Inactivation Kinetics
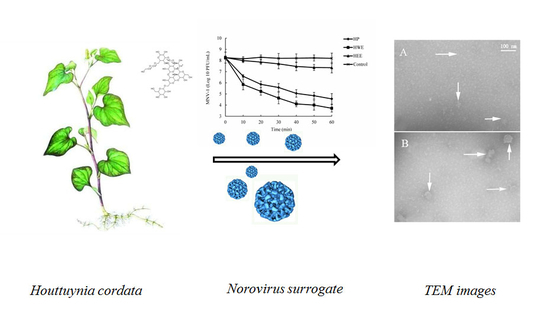
2.6. Effect of HP on MNV-1 Viral Particles
3. Discussion
4. Materials and Methods
4.1. Preparation of HP, HWE, and HEE
4.2. Chemical Analysis
4.3. Propagation of Viruses
4.4. Antiviral Effects of HP, HWE, and HEE
4.5. Plaque Assay
4.6. Transmission Electron Microscopy (TEM)
4.7. Viral Inactivation Kinetics
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HP | Houttuynia cordata polysaccharide |
| HWE | Houttuynia cordata water extract |
| HEE | Houttuynia cordata ethanol extract |
| GlaUA | galacturonic acid |
| Gal | galactose |
| Rha | rhamnose |
| Ara | arabinose |
| GluA | glucuronic acid |
| Glc | glucose |
| Man | mannose |
| Xyl | xylose |
| HuNoV | Human norovirus |
| MNV-1 | murine norovirus-1 |
| ATCC | American Type Culture Collection |
| PFU | Plaque forming units |
References
- Yang, L.; Jiang, J.G. Bioactive components and functional properties of Hottuynia cordata and its applications. Pharm. Biol. 2009, 47, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Kamiya, M.; Hayashi, T. Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med. 1995, 61, 237–241. [Google Scholar] [CrossRef]
- Karst, S.M.; Wobus, C.E.; Goodfellow, I.G.; Green, K.Y.; Virgin, H.W. Advances in norovirus biology. Cell Host Microbe 2014, 15, 668–680. [Google Scholar] [CrossRef]
- Jones, M.K.; Grau, K.R.; Costantini, V.; Kolawole, A.O.; De Graaf, M.; Freiden, P.; Graves, C.L.; Koopmans, M.; Wallet, S.M.; Tibbetts, S.A.; et al. Human norovirus culture in B cells. Nat. Protoc. 2015, 10, 1939–1942. [Google Scholar] [CrossRef]
- Li, D.; Baert, L.; Uyttendaele, M. Inactivation of food-borne viruses using natural biochemical substances. Food Microbiol. 2013, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Peng, T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antivir. Res. 2013, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zakay-Rones, Z.; Thom, E.; Wollan, T.; Wadstein, J. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. J. Int. Med. Res. 2004, 32, 132–140. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbial. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Yang, M.; Lee, G.; Si, J.; Lee, S.J.; You, H.J.; Ko, G. Curcumin shows antiviral properties against norovirus. Molecules 2016, 21, 1401. [Google Scholar] [CrossRef] [PubMed]
- Wobus, C.E.; Thackray, L.B.; Virgin, H.W. Murine norovirus: A model system to study norovirus biology and pathogenesis. J. Virol. 2006, 80, 5104–5112. [Google Scholar] [CrossRef]
- Wang, D.; Tian, P. Inactivation conditions for human norovirus measured by an in situ capture-qRT-PCR method. Int. J. Food Microbiol. 2014, 172, 76–82. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, Y.; Guo, C.; Yang, X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polym. 2011, 83, 537–544. [Google Scholar] [CrossRef]
- Cheng, B.H.; Chan, J.Y.W.; Chan, B.C.L.; Lin, H.Q.; Han, X.Q.; Zhou, X.; Wan, D.C.C.; Wang, Y.F.; Leung, P.C.; Fung, K.P.; et al. Structural characterization and immunomodulatory effect of a polysaccharide HCP-2 from Houttuynia cordata. Carbohydr. Polym. 2014, 103, 244–249. [Google Scholar] [CrossRef]
- Kafle, K.; Park, Y.B.; Lee, C.M.; Stapleton, J.J.; Kiemle, S.N.; Cosgrove, D.J.; Kim, S.H. Effects of mechanical stretching on average orientation of cellulose and pectin in onion epidermis cell wall: A polarized FT-IR study. Cellulose 2017, 24, 3145–3154. [Google Scholar] [CrossRef]
- Shakhmatov, E.G.; Udoratina, E.V.; Atukmaev, K.V.; Makarova, E.N. Extraction and structural characteristics of pectic polysaccharides from Abies sibirica L. Carbohydr. Polym. 2015, 123, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.W. Food Carbohydrates: Chemistry, Physical Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Acharya, D.; Mitaine-Offer, A.C.; Kaushik, N.; Miyamoto, T.; Paululat, T.; Lacaille-Dubois, M.A. Furostane-Type Steroidal Saponins from the Roots of Chlorophytum borivilianum. Helv. Chim. Acta 2008, 91, 2262–2269. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, J.; Tang, Q.; Liu, Y.; Zhang, A.; Pan, Y. Structural elucidation of a neutral fucogalactan from the mycelium of Coprinus comatus. Carbohydr. Res. 2006, 341, 1130–1134. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Isolation and structural characterization of protopectin from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr. Polym. 2005, 60, 205–213. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, W.; Kim, M.; Choi, E.H.; Kim, Y.J. Arc discharge-mediated disassembly of viral particles in water. Water Res. 2016, 102, 305–312. [Google Scholar] [CrossRef]
- Kang, C.G.; Hah, D.S.; Kim, C.H.; Kim, Y.H.; Kim, E.; Kim, J.S. Evaluation of antimicrobial activity of the methanol extracts from 8 traditional medicinal plants. Toxicol. Res. 2011, 27, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, L.; Wu, H.; Chen, S.; Zhu, Q.; Gao, H.; Yu, X.; Wang, Y.; Su, W.; Yao, X.; et al. Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuynia cordata Thunb. Antivir. Res. 2017, 144, 273–280. [Google Scholar] [CrossRef]
- Eom, S.H.; Moon, S.Y.; Lee, D.S.; Kim, H.J.; Park, K.; Lee, E.W.; Kim, T.H.; Chung, Y.H.; Lee, M.S.; Kim, Y.M. In vitro antiviral activity of dieckol and phlorofucofuroeckol-A isolated from edible brown alga Eisenia bicyclis against murine norovirus. Algae 2015, 30, 241–248. [Google Scholar] [CrossRef]
- Józefiak, D.; Rutkowski, A.; Martin, S.A. Carbohydrate fermentation in the avian ceca: A review. Anim. Feed Sci. Tech. 2004, 113, 1–15. [Google Scholar] [CrossRef]
- Ferrazzano, G.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef]
- Seo, K.; Lee, J.E.; Lim, M.Y.; Ko, G. Effect of temperature, pH, and NaCl on the inactivation kinetics of murine norovirus. J. Food Prot. 2012, 75, 533–540. [Google Scholar] [CrossRef]
- Bozkurt, H.; D’Souza, D.H.; Davidson, P.M. A comparison of the thermal inactivation kinetics of human norovirus surrogates and hepatitis A virus in buffered cell culture medium. Food Microbiol. 2014, 42, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453, 1–9. [Google Scholar] [CrossRef]
- Lipson, S.M.; Sethi, L.; Cohen, P.; Gordon, R.E.; Tan, I.P.; Burdowski, A.; Stotzky, G. Antiviral effects on bacteriophages and rotavirus by cranberry juice. Phytomedicine 2007, 14, 23–30. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, S.Y.; Oh, M.; Seok, J.H.; Kim, S.; Chung, Y.B.; Gowda, K.G.; Mun, J.Y.; Chung, M.S.; Kim, K.H. Antiviral effects of black raspberry (Rubus coreanus) seed extract and its polyphenolic compounds on norovirus surrogates. Biosci. Biotechnol. Biochem. 2016, 80, 1196–1204. [Google Scholar] [CrossRef]
- Pang, J.; Dong, W.; Li, Y.; Xia, X.; Liu, Z.; Hao, H.; Jiang, L.; Liu, Y. Purification of houttuynia cordata thunb. essential oil using macroporous resin followed by microemulsion encapsulation to improve its safety and antiviral activity. Molecules 2017, 22, 293. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lu, X.; Ling, L.; Li, H.; Ou, Y.; Shi, X.; Lu, Y.; Zhang, Y.; Chen, D. Houttuynia cordata polysaccharides ameliorate pneumonia severity and intestinal injury in mice with influenza virus infection. J. Ethnopharmacol. 2018, 218, 90–99. [Google Scholar] [CrossRef]
- Kumar, M.; Prasad, S.K.; Hemalatha, S. A current update on the phytopharmacological aspects of Houttuynia cordata Thunb. Pharmacogn. Rev. 2014, 8, 22–35. [Google Scholar] [PubMed] [Green Version]
- Chen, S.D.; Gao, H.; Zhu, Q.C.; Wang, Y.Q.; Li, T.; Mu, Z.Q.; Wu, H.L.; Peng, T.; Yao, X.S. Houttuynoids A–E, anti-herpes simplex virus active flavonoids with novel skeletons from Houttuynia cordata. Org. Lett. 2012, 14, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Sekita, Y.; Murakami, K.; Yumoto, H.; Mizuguchi, H.; Amoh, T.; Ogino, S.; Matsuo, T.; Miyake, Y.; Fukui, H.; Kashiwada, Y. Anti-bacterial and anti-inflammatory effects of ethanol extract from Houttuynia cordata poultice. Biosci. Biotechnol. Biochem. 2016, 80, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Sun, L.; Chen, J.; Zhong, J.; Zhang, Y.; Zhang, R. A novel polysaccharide conjugate from bullacta exarata induces G1-phase arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Molecules 2017, 22, 384. [Google Scholar] [CrossRef]
- Chen, X.; Song, L.; Wang, H.; Liu, S.; Yu, H.; Wang, X.; Li, R.; Liu, T.; Li, P. Partial Characterization, the Immune Modulation and Anticancer Activities of Sulfated Polysaccharides from Filamentous Microalgae Tribonema sp. Molecules 2019, 24, 322. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.; Chen, M.; Crawford, R.; Ivanova, E. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Jaafar, H.Z. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 2010, 15, 6244–6256. [Google Scholar] [CrossRef]
- Liu, D.; Liao, N.; Ye, X.; Hu, Y.; Wu, D.; Guo, X.; Zhong, J.; Wu, J.; Chen, S. Isolation and structural characterization of a novel antioxidant mannoglucan from a marine bubble snail, Bullacta exarata (Philippi). Mar. Drugs 2013, 11, 4464–4477. [Google Scholar] [CrossRef]
- Julian, T.R.; Trumble, J.M.; Schwab, K.J. Evaluating efficacy of field-generated electrochemical oxidants on disinfection of fomites using bacteriophage MS2 and mouse norovirus MNV-1 as pathogenic virus surrogates. Food Environ. Virol. 2014, 6, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Schwab, K.J. Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl. Environ. Microb. 2008, 74, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Sangster, M.Y.; D’Souza, D.H. In vitro effects of pomegranate juice and pomegranate polyphenols on foodborne viral surrogates. Foodborne Pathog. Dis. 2010, 7, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Skaba, D.; Czuba, Z.P.; Krol, W. Inhibition of inflammatory mediators by neobavaisoflavone in activated RAW264.7 macrophages. Molecules 2011, 16, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Hermelink, A.; Naumann, D.; Piesker, J.; Lasch, P.; Laue, M.; Hermann, P. Towards a correlative approach for characterising single virus particles by transmission electron microscopy and nanoscale Raman spectroscopy. Analyst 2017, 142, 1342–1349. [Google Scholar] [CrossRef]
Sample Availability: Not Available. |





| Composition b | Extract/Fraction | ||
|---|---|---|---|
| HP | HWE | HEE | |
| Extraction yield (%) | 5.73 ± 2.16 | 16.31 ± 1.64 | 9.63 ± 2.31 |
| Total phenolic (μg GAE/mg) | ND c | 14.59 ± 2.41 | 25.42 ± 3.17 |
| Total flavonoid (μg RE/mg) | ND | 10.62 ± 1.43 | 19.76 ± 3.73 |
| Protein (%) | 0.89 ± 0.12 | 2.54 ± 0.12 | 5.68 ± 1.76 |
| Total carbohydrate (%) | 81.12 ± 5.98 | 38.01 ± 3.98 | 2.37 ± 1.45 |
| Neutral sugars (%) | 56.33 ± 4.37 | 26.58 ± 2.54 | 2.37 ± 1.45 |
| Uronic acid (%) | 23.77 ± 3.42 | 12.34 ± 2.13 | ND |
| Molar ratio of monosaccharides d | |||
| GlaUA | 1.56 | 1.37 | ND |
| Gal | 1.49 | 0.94 | ND |
| Rha | 0.83 | ND | ND |
| Ara | 0.68 | ND | ND |
| GluA | 0.31 | ND | ND |
| Glc | 1.26 | 0.31 | ND |
| Man | 0.14 | ND | ND |
| Xyl | 1.11 | ND | ND |
| Sugar a | Partially O-methylated Alditol Acetates | Molar Ratio | Linkage d |
|---|---|---|---|
| GalpA | 2,3,6-Me3 Gal-6-d2 b | 1.74 | →4)-GalpA-(1→ |
| Galp | 2,3,6-Me3 Gal | 0.93 | →4)-Galp-(1→ |
| 2,3,4-Me3 Gal | 0.14 | →6)-Galp-(1→ | |
| 2,3-Me2 Gal | 0.21 | →4,6)-Galp-(1→ | |
| 2,3,4,6-Me4 Gal | 0.35 | Galp-(1→ | |
| Glcp | 2,3,6-Me3 Glc | 0.95 | →4)-Glcp-(1→ |
| 2,3,4,6-Me4 Glc c | 0.37 | Glcp-(1→ | |
| Xylp | 2,3-Me2 Xyl | 0.84 | →4)-Xylp-(1→ |
| Rhap | 3-Me Rha | 0.22 | →2,4)-Rhap-(1→ |
| Araf | 2,3-Me2 Ara | 0.17 | →5)-Araf-(1→ |
| H. cordata Extracts | CC50 (μg/mL) a | EC50 (μg/mL) b | SI c |
|---|---|---|---|
| HEE | 2132.43 ± 426.17 | 1095.53 ± 113.43 | 1.95 ± 0.84 |
| HWE | 1237.52 ± 367.65 * | 76.75 ± 17.89 * | 16.14 ± 3.81 * |
| HP | 1074.76 ± 187.31 * | 187.24 ± 77.82 * | 5.74 ± 1.96 * |
| Extracts | Concentration | MNV-1 (log10 PFU/mL) | |||
|---|---|---|---|---|---|
| Low Count (4.38 log10) | High Count (8.09 log10) | ||||
| Recovered Titer c | Reduction e | Recovered Titer | Reduction | ||
| PBS a | 0 μg/mL | 4.38 ± 1.71 | 0 | 8.09 ± 1.95 | 0 |
| 2TU b | 50 μM | 2.50 ± 0.83* | 1.88 | 4.33 ± 2.14 * | 3.76 |
| HP | 100 μg/mL | 3.77 ± 1.04 | 0.61 | 7.49 ± 2.53 | 0.60 |
| 250 μg/mL | 2.62 ± 1.03 * | 1.76 | 6.86 ± 1.81 * | 1.23 | |
| 500 μg/mL | ND* d | 4.38 | 4.61 ± 1.72 * | 3.48 | |
| HWE | 100 μg/mL | 2.51 ± 0.53 * | 1.87 | 6.71 ± 2.12 * | 1.38 |
| 250 μg/mL | ND * | 4.38 | 6.26 ± 1.32 * | 1.83 | |
| 500 μg/mL | ND * | 4.38 | 3.96 ± 0.74 * | 4.12 | |
| HEE | 100 μg/mL | 4.21 ± 0.87 | 0.16 | 8.03 ± 2.89 | 0.06 |
| 250 μg/mL | 3.48 ± 1.33 * | 0.90 | 7.52 ± 1.73 | 0.57 | |
| 500 μg/mL | 3.24 ± 0.41 * | 1.14 | 7.01 ± 2.07 * | 0.98 | |
| HP (μg/mL) | Weibull Model Parameters a | ||||
|---|---|---|---|---|---|
| K ± SD b | α ± SD | D-Value (min) ± SD | RMSE | R2 | |
| 100 | 0.144 ± 0.65 | 0.581 ± 0.04 | 28.098 ± 2.44 | 0.03 | 0.97 |
| 250 | 0.213 ± 0.01 | 0.817 ± 0.26 | 6.647 ± 0.93 | 0.02 | 0.98 |
| 500 | 0.866 ± 0.08 | 0.308 ± 0.04 | 1.597 ± 0.31 | 0.04 | 0.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, D.; Sun, L.; Zou, S.; Chen, J.; Mao, H.; Zhang, Y.; Liao, N.; Zhang, R. Antiviral Effects of Houttuynia cordata Polysaccharide Extract on Murine Norovirus-1 (MNV-1)—A Human Norovirus Surrogate. Molecules 2019, 24, 1835. https://doi.org/10.3390/molecules24091835
Cheng D, Sun L, Zou S, Chen J, Mao H, Zhang Y, Liao N, Zhang R. Antiviral Effects of Houttuynia cordata Polysaccharide Extract on Murine Norovirus-1 (MNV-1)—A Human Norovirus Surrogate. Molecules. 2019; 24(9):1835. https://doi.org/10.3390/molecules24091835
Chicago/Turabian StyleCheng, Dongqing, Liang Sun, Songyan Zou, Jiang Chen, Haiyan Mao, Yanjun Zhang, Ningbo Liao, and Ronghua Zhang. 2019. "Antiviral Effects of Houttuynia cordata Polysaccharide Extract on Murine Norovirus-1 (MNV-1)—A Human Norovirus Surrogate" Molecules 24, no. 9: 1835. https://doi.org/10.3390/molecules24091835
APA StyleCheng, D., Sun, L., Zou, S., Chen, J., Mao, H., Zhang, Y., Liao, N., & Zhang, R. (2019). Antiviral Effects of Houttuynia cordata Polysaccharide Extract on Murine Norovirus-1 (MNV-1)—A Human Norovirus Surrogate. Molecules, 24(9), 1835. https://doi.org/10.3390/molecules24091835